Speeding up the hydrogen production by the magic topological surface states
Water electrolysis could provide high-quality hydrogen gas that can be used in fuel cells directly. However, since noble metals, such as platinum and iridium, are currently needed to initiate such a reaction, the cost is very high. "Obviously, catalysts that are low-cost with high-activity are needed to make hydrogen energy more competitive with traditional technologies," says Guowei Li at the Max Planck Institute for Chemical Physics of Solids, who studied the surface reactions of several topological materials.
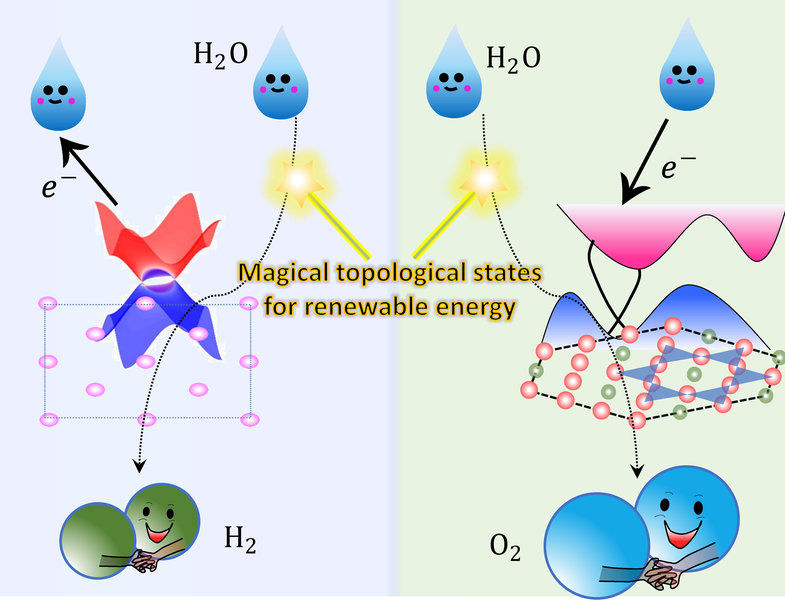
Topological non-trivial surface states can accept or donate electrons during the water electrolysis process.
© MPI CPfS
It was obviously a great challenge to find alternatives beyond noble metals. "Topology may be the key to unlocking the barrier in the search for ideal catalysts," says Prof. Claudia Felser, director at the Max Planck Institute for Chemical Physics of Solids. "We studied the surface properties of materials with topological order, from topological insulators to topological semimetals and metals, all these materials have non-trivial surface states that are protected by symmetries"
"In other words, these surface states are very stable and robust against surface modifications such as impurity scattering and even oxidation: the question we're asking is can we find such a perfect system that combines topological order, lost-cost, high efficiency, and high stability."
The team from the Max Planck Institute Chemical Physics of Solids, Dresden together with colleagues from the TU Dresden and the Max Planck Institute for Microstructure Physics and Max-Planck-Institut für Kohlenforschung, Mülheim published a breakthrough result in Science Advances concerning a topological material, namely a magnetic Weyl-semimetal, that is a superior oxygen evolution reaction (OER) catalyst. The magnetic weyl semimetal that the team identified is Co3Sn2S2, a Kagome-lattice Shandite compound.[1]
High-quality bulk single crystals of Co3Sn2S2with sizes of up to centimeters can be exfoliated into thin-layers with defined crystal surfaces. The team showed that these surfaces act as superior catalysts for water splitting, even though the surface area is several orders of magnitudes smaller than today´s conventional nano-structured catalysts. In collaboration with Yan Sun's theory group from the Max Planck Institute Chemical Physics of Solids, they found that there are cobalt-derived topological surface states just above the Fermi level. In the water oxidation process, these surface states can accept electrons from the reaction intermediates, acting as an electron channel whose resistance is not affected by the harsh electrochemical environment.
Inspired by this strategy, the team then investigated the catalytic performance of a Dirac nodal arc semimetal PtSn4, a compound that has much lower percentage of expensive platinum.[2] Such crystals showed superior electrocatalytic stability for periods of time exceeding one month.
"The work serves as an interesting lens into the chemistry of these reaction processes and could be a pathway towards understanding the chemistry itself by clear knowledge of the topological nature of the semimetal catalyst," says one of the expert reviewers of the paper.
Original publication
[1] G. Li, Q. Xu, W. Shi, C. Fu, L. Jiao, M. E. Kamminga, M. Yu, H. Tüysüz, N. Kumar, V. Süß, R. Saha, A. K. Srivastava, S. Wirth, G. Auffermann, J. Gooth, S. Parkin, Y. Sun, E. Liu, C. Felser; "Surface states in bulk single crystal of topological semimetal Co3Sn2S2 toward water oxidation"; Sci. Adv.; 2019, 5, eaaw9867.
[2] G. Li, C. Fu, W. Shi, L. Jiao, J. Wu, Q. Yang, R. Saha, M. E. Kamminga, A. K. Srivastava, E. Liu, A. N. Yazdani, N. Kumar, J. Zhang, G. R. Blake, X. Liu, M. Fahlman, S. Wirth, G. Auffermann, J. Gooth, S. Parkin, V. Madhavan, X. Feng, Y. Sun, C. Felser; "Dirac Nodal Arc Semimetal PtSn4: An Ideal Platform for Understanding Surface Properties and Catalysis for Hydrogen Evolution"; Angewandte Chemie; 2019, 58, 2-10.
Original publication
[1] G. Li, Q. Xu, W. Shi, C. Fu, L. Jiao, M. E. Kamminga, M. Yu, H. Tüysüz, N. Kumar, V. Süß, R. Saha, A. K. Srivastava, S. Wirth, G. Auffermann, J. Gooth, S. Parkin, Y. Sun, E. Liu, C. Felser; "Surface states in bulk single crystal of topological semimetal Co3Sn2S2 toward water oxidation"; Sci. Adv.; 2019, 5, eaaw9867.
[2] G. Li, C. Fu, W. Shi, L. Jiao, J. Wu, Q. Yang, R. Saha, M. E. Kamminga, A. K. Srivastava, E. Liu, A. N. Yazdani, N. Kumar, J. Zhang, G. R. Blake, X. Liu, M. Fahlman, S. Wirth, G. Auffermann, J. Gooth, S. Parkin, V. Madhavan, X. Feng, Y. Sun, C. Felser; "Dirac Nodal Arc Semimetal PtSn4: An Ideal Platform for Understanding Surface Properties and Catalysis for Hydrogen Evolution"; Angewandte Chemie; 2019, 58, 2-10.
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.