Superfluorescent emission in the UV range
Free-electron laser FLASH coaxes superfluorescent emission from the noble gas xenon
Advertisement
Scientists have for the first time induced superfluorescence in the extreme ultraviolet range. Superfluorescence, or superradiance, could be used to build a laser that does not require an optical resonator. The team headed by DESY’s lead scientist Nina Rohringer used DESY’s free-electron laser FLASH to stimulate xenon, a noble gas, inside a narrow tube, causing it to emit coherent radiation, like a laser.
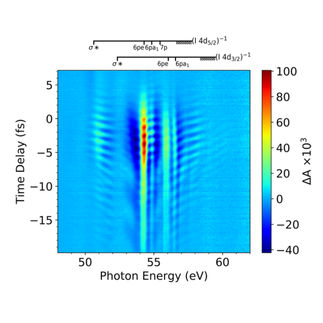
The xenon superfluorescence shows up as a bright line (yellow) superimposed on the averaged free-electron laser spectrum (purple, averaged over many shots).
European XFEL, Laurent Mercadier
“The phenomenon of superfluorescence was first discovered in the microwave range in the 1970s, and then demonstrated for infrared and optical wavelengths too,” explains Rohringer. “In the meantime, superfluorescence has also been observed in the X-ray domain, and at one time this mechanism was believed to be a promising candidate for building X-ray lasers. Until now, however, superfluorescence had not been demonstrated in the extreme ultraviolet, or XUV, range.”
In superfluorescence, the incident light is amplified and emitted along the axis of the medium as a narrow beam of coherent radiation, like in a laser. To produce superfluorescence in the XUV spectrum, the incoming light needs to have enough energy to knock the electrons out of the inner shell of the atoms that make up the lasing medium. Redistribution within the electron shell (Auger decay) leads to a situation in which more particles find themselves in an excited state than in an unexcited state. Physicists refer to this as population inversion.
When an atom drops from an excited to a lower energy state, it emits a photon. If enough atoms undergo population inversion, a small number of isolated decays of the excited states of individual atoms produce a strongly correlated quantum mechanical state, known as entanglement, so that the entire ensemble of excited atoms drops to a lower energy level in unison (coherently), emitting a high-intensity pulse of light.
The simultaneous emission is caused by a quantum mechanical effect, which is however expressed macroscopically: the entangled atoms are not individually coupled to the quantised light field (vacuum fluctuations in the light field), but as a single, overall medium.
The team of Nina Rohringer from DESY, Laurent Mercadier from the European XFEL and José Ramon Crespo López-Urrutia from the Max Planck Institute of Nuclear Physics in Heidelberg fired intense, used the CAMP scientific instrument to focus pulses from FLASH into a cell filled with xenon gas, to produce a plasma channel 4.5 millimetres long and 0.04 millimetres in diameter. The high-energy pulses, having a wavelength of 13 nanometres (millionths of a millimetre), produced a strong population inversion in the gas, which discharged after about a tenth of a nanosecond to emit a bright, laser pulse lasting 7 to 21 trillionths of a second (picoseconds) and having wavelengths of 65 and 68 nanometres.
“Although we used a free-electron laser to excite the system, able to provide particularly bright and short pulse, we were able to show that the excitation does not necessarily have to be produced by free-electron laser light in order to produce coherent superfluorescent emission,” explains Rohringer. In the form demonstrated, xenon’s superfluorescence should not be viewed as a rival to free-electron lasers or other XUV lasers, the scientists write. However, the duration of the pulse could still be shortened and, in contrast to free-electron lasers, the radiation produced using superfluorescence consists of sharply defined spectral lines with a precisely determined wavelength, which makes it interesting for certain methods of analysis, such as spectroscopy. Most of all, however, this research leads to a better understanding of the process of superfluorescence – a macroscopic quantum phenomenon.
Original publication
"Evidence of Extreme Ultraviolet Superfluorescence in Xenon"; L. Mercadier, A. Benediktovitch, C. Weninger, M. A. Blessenohl, S. Bernitt, H. Bekker, S. Dobrodey, A. Sanchez-Gonzalez, B. Erk, C. Bomme, R. Boll, Z. Yin, V. P. Majety, R. Steinbrügge, M. A. Khalal, F. Penent, J. Palaudoux, P. Lablanquie, A. Rudenko, D. Rolles, J. R. Crespo López-Urrutia, and N. Rohringer; Physical Review Letters; 2019I: