Scientists offer designer 'big atoms' on demand
In the not-so-distant future, researchers may be able to build atoms to your specifications with the click of a button. It's still the stuff of science fiction, but a team at the University of Colorado Boulder reports that it is getting closer when it comes to controlling and assembling particles called "big atoms."
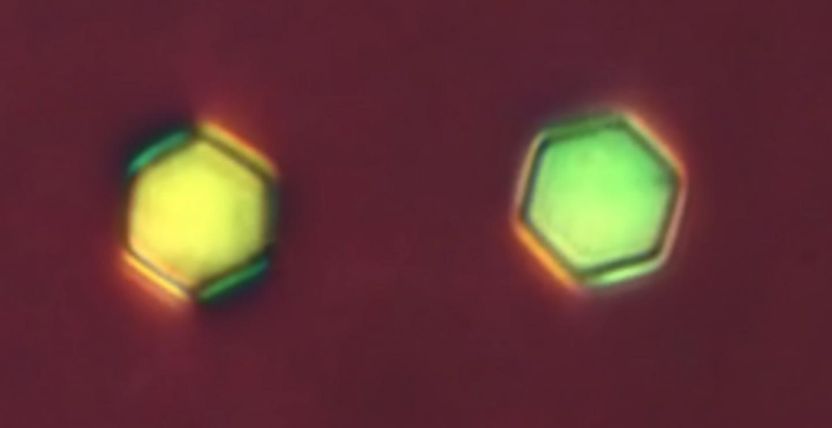
These hexagonally-shaped grains of silica, when suspended in a liquid crystal, may resemble elements in the periodic table.
Smalyukh lab
The new research, which will be published in Nature, centers around colloidal particles that, when mixed with liquid crystals, act a lot like the elements of the periodic table. These particles give physicists the opportunity to probe how hydrogen, helium and other atoms behave and interact without needing to zoom down to the atomic level.
By exposing the big atoms to different kinds of light, for example, the team showed that it could flip their charges with a flick of a switch. In other words, particles that once attracted each other now repel each other.
"Because we have so much control, we have the ability to design how these particles assemble and what properties they have," said Ivan Smalyukh, a professor in the Department of Physics. "It's like a designer toolkit."
That designer toolkit begins with a simple ingredient: liquid crystals.
These materials, which provide the crisp images on your smartphone screen, are often made up of molecules in tidy arrangements, such as rods that all point in a single direction.
In the last decade or so, however, scientists have noticed something odd about these fluid-like materials. If you drop particles, such as microscopic grains of silica, into liquid crystals, the once orderly molecules inside will bend and squish to make room for the new additions--a bit like shoving a football lineman into an already-crowded subway car.
And, remarkably, the way those liquid crystals bend can be mathematically analogous to the structures of atoms' electron shells.
"How the liquid crystals bend around the particles is very important," said Smalyukh, also in the Materials Science Engineering Program and Department of Electrical, Computer, and Energy Engineering. "When you disrupt those molecules, it costs energy, and that energy drives interesting interactions."
Bend liquid crystal molecules in just the right way and the bits of silica will clink into each other as if they were two atoms bonding together, but much bigger.
The problem, Smalyukh said, is that until recently, scientists had very little control over those big atom interactions. His group had the solution.
To make their unique colloidal mixture, Smalyukh and his colleagues used pieces of silica in the shape of hexagons for their big atoms. But before plopping those particles into liquid crystals, the researchers coated them in a type of dye that rotates when exposed to different kinds of light.
When the researchers exposed their mixture to a certain type of blue light, the liquid crystal molecules would bend around the hexagons following one pattern. Use a different kind of light and they would bend in an entirely different way.
The group reported that they could switch a big atom's effective charge from positive to negative and back again on a whim.
"It's almost like you can shine light and turn matter into antimatter," said Ye Yuan, a postdoctoral researcher in physics and lead author of the new study. Other coauthors included postdocs Qingkun Liu and Bohdan Senyuk.
And, Yuan said, the team was able to control those interactions using an ordinary lamp with a filter on it--no high-powered lasers required.
"In principle, we could have a good sunny day in Colorado and bring our samples outside and see those interactions," Yuan said.
Which gets him excited for what the team could build with these big atoms. The researchers believe that, with the right tweaks, they could use their method to assemble particles in unique ways, creating faux-atomic structures that don't exist in nature--then dissolve those structures just as easily.
"In some ways, we still need to figure out what we can do with this," Smalyukh said.
Building your own periodic table from scratch? Stay tuned.
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Bonfiglioli Vectron GmbH - Krefeld, Germany
Why carbon nanotubes spell trouble for cells