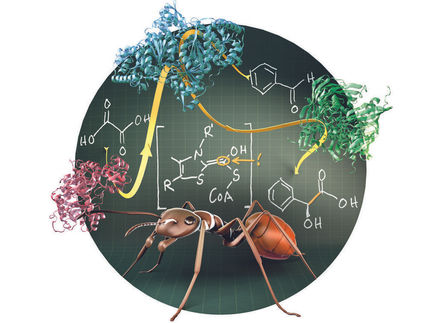
Development of an Efficient Enantioselective Desymmetrization Strategy Using Recombinant Pig Liver Esterases
An economically significant process intensification for the efficient enzymatic synthesis of (1S,2R)-1-(methoxycarbonyl) cyclohex-4-ene-2-carboxylic acid was achieved by a careful adaption of the reaction mode to minimize mass transfer limitations.

Enzymicals AG

Figure 1 (1S,2R)-Monoester is a key intermediate for biologically active molecules.
Enzymicals AG
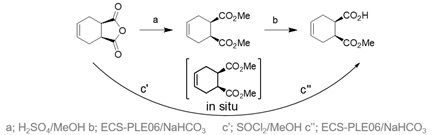
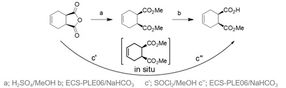
Figure 2 Conventional (a,b) and sequential multistep one-pot reaction (c’, c’’) synthesis pathway to (1S,2R)-monoester
Enzymicals AG

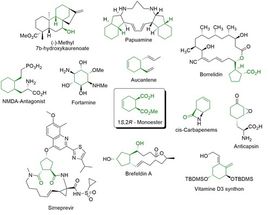
(1S,2R)-1-(methoxycarbonyl) cyclohex-4-ene-2-carboxylic acid (1S,2R-Monoester) is a valuable key intermediate for various biologically active molecules (Figure 1). This compound was recently established as an Enzymicals catalogue product after development of an efficient biocatalytic sequential multistep one-pot reaction.
The initial synthesis was established after the development of a novel biocatalytic sequential multistep one-pot reaction.[1] Herein the main cost drivers were eliminated by switching the reaction mode to a chemoenzymatic reaction concept, which simplified downstream processing and improved isolated yield (Figure 2). The process had been well-characterized in terms of pH and temperature optimum, and full conversions with an excellent enantiomeric excess (> 99.5 %) were achieved using the recombinant pig liver esterase ECS-PLE06, which is also available at Enzymicals.
Further developments in collaboration with two academic partners focused on the precise influence of mass transfer of the poorly water-soluble substrate, which was not considered in detail by the earlier studies. The main aim was to enhance the overall productivity of the process and bring it to industrial requirements for a batch process. For this purpose, a newly-developed reaction trajectory analysis methodology was applied, which facilitated a strong reduction of the main limiting parameters. As result the biocatalytic synthesis of the target monoester was enhanced to 0.4 M (75 g·L−1) batches of product with an biocatalyst yield and productivity of 4.36 g·gbiocat−1 and 20.2 g·L−1·h−1.[2]
This successful process optimization is a result of a fruitful collaboration of Enzymicals AG, Dr. Jan von Langermann, Biocatalytic Synthesis group at University of Rostock and Prof. John M. Woodley, Department of Chemical and Biochemical Engineering at Technical University of Denmark.
Original publication
[1] Süss P., Borchert S., Hinze J., Illner S., v. Langermann J., Kragl U., Bornscheuer U.T., Wardenga R.; "Chemoenzymatic Sequential Multistep One-Pot Reaction for the Synthesis of (1S,2R)-1-(Methoxycarbonyl)cyclohex-4-ene-2-carboxylic Acid with Recombinant Pig Liver Esterase"; Org. Process Res. Dev.; 2015, 19 (12), pp 2034–2038.
[2] Meissner M.P., Süss P., Brundiek H., Woodley J.M., v. Langermann J.; "Scoping the Enantioselective Desymmetrization of a Poorly Water-Soluble Diester by Recombinant Pig Liver Esterase"; Org. Process Res. Dev.; 2018, 22, 1518−1523.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.