Nanomicrocell catalysts: A new kind of highly efficient integrated catalyst system
chemical reactions are involving directional migration of electrons and charged intermediates, depending on the redox potentials of different reactive sites. The chemical energy can be converted into electrical energy when the chemical reactions are occurred in fuel cell devices. However, restricted by the high energy barriers of chemical reactions, numerous chemical reactions have to be performed under harsh conditions or in the presence catalysts by changing the reaction paths, even though the Gibbs free energy is much less than zero. Recently, a new kind of integrated catalyst system, denoted as nanomicrocell catalysts, was developed, which opened a new window for development of highly efficient catalysts by markedly reducing the energy barriers of chemical reactions.
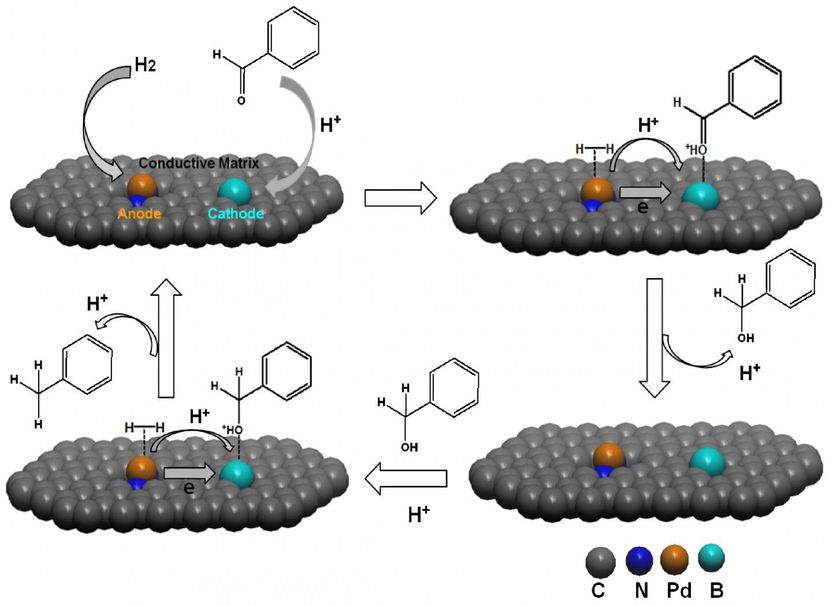
Proposed catalytic cycles for hydrogenation reduction of benzaldehyde catalyzed by the nanomicrocell catalyst Pd-BNCD.
©Science China Press
This work was reported in Science Bulletin by Chuan-De Wu et al. from Zhejiang University. Inspired by the natural phenomena, such as steel corrosion, the authors proposed a new kind of integrated catalyst system, denoted as nanomicrocell catalysts, consisting of different redox-active sites with different oxidation-reduction potentials and catalytic properties immobilized on conductive matrices. Similar to traditional fuel cells, the nanomicrocell catalyst system integrates paired anodes and cathodes that are connected by nanosized conductive matrices, which could significantly improve the catalytic efficiency in chemical reactions.
The authors prepared a nanomicrocell catalyst, consisting of boron-and-nitrogen co-doped carbon nanodots (denoted as BNCDs) and immobilized Pd species by coordinating with N atoms, to study the catalytic properties of the proof-of-concept catalyst system. In the nanomicrocell catalyst, BNCDs were used as conductive matrices, immobilized Pd species on N sites as anode and electron-deficient B atoms as cathode. The authors selected catalytic hydrogenation of benzaldehyde as a model reaction to study the roles of different constituent elements in the catalyst system on the catalytic properties. Detailed experimental results showed that the catalytic properties of the nanomicrocell catalyst in hydrogenation of benzaldehyde are highly depending on the selective adsorption and catalytic activation of different reactants by different redox-active electrodes, and transportation efficiency of electrons and charge carriers.
According to the results obtained in this work, the nanomicrocell catalyst system provides a new perspective to illustrate many difficultly understood phenomena in catalytic reactions, and opens up a new window for the designed synthesis of advanced catalysts to improve the catalytic properties by markedly reducing chemical reaction energy barriers. The nanomicrocell catalyst system also provides new way for development of inhibitory catalysts to decelerate chemical reactions in corrosion protection field, and preparation of efficient electrocatalysts and photocatalysts.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.



























































