First astrophysical detection of the helium hydride ion
Advertisement
HeH+ was the first molecule that formed when, almost 14 billion years ago, falling temperatures in the young Universe allowed recombination of the light elements produced in the Big Bang. At that time, ionized hydrogen and neutral helium atoms reacted to form HeH+.
Despite its importance in the history of the early Universe, HeH+ has so far escaped detection in astrophysical nebulae. Operating the GREAT far-infrared spectrometer onboard the flying observatory SOFIA, an international research team led by Rolf Güsten from the Max-Planck-Institut für Radioastronomie (MPIfR) in Bonn, Germany, reports the unambiguous detection of the molecule towards the planetary nebula NGC 7027.
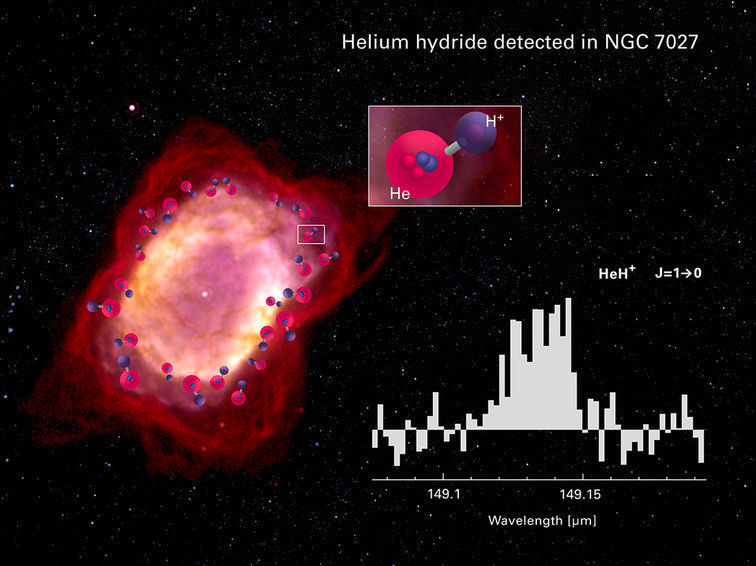
Spectrum of HeH+ as observed with GREAT on board of SOFIA towards the planetary nebula NGC 7027. In the underlying image from the Hubble/NICMOS camera, the sharp transition zone between the ionized HII region (white-yellow) and the cool envelope (red colour) is nicely visible. It is in this ionization front where HeH+ is formed (marked by an artist’s concept of the molecular structure). The sky area covered by the GREAT instrument with a size of 14.3 arcsec includes most of the nebula’s emission. The spectral line width of the HeH+ profile is determined by the motion of the expanding envelope.
© Composition: NIESYTO design; Image NGC 7027: William B. Latter (SIRTF Science Center/Caltech) and NASA/ESA; Spectrum: Rolf Güsten/MPIfR (Nature, April 18, 2019)
During the dawn of chemistry when the temperature in the young Universe had fallen below ~4000 K, the ions of the light elements (hydrogen, helium, deuterium and traces of lithium) produced in Big Bang nucleosynthesis recombined in reverse order of their ionization potential. Helium combined first with free electrons to form the first ever neutral atom. At that time hydrogen was still ionized (present in form of bare protons). Helium atoms combined with these protons into the helium hydride ion HeH+, the Universe’s first molecular bond. As recom¬bi¬nation progressed, HeH+ reacted with then neutral hydrogen and created a first path to the forma¬tion of molecular hydrogen - marking the beginning of the modern Universe.
Despite its unquestioned importance in the history of the early Universe, the HeH+ molecule has so far escaped detection in interstellar space. Studied in the laboratory as long ago as 1925, dedicated searches during the last decades have been unsuccessful, thereby challenging our understanding of the underlying chemical networks. “The chemistry of the Universe began with HeH+. The lack of definitive evidence of its very existence in interstellar space has been a dilemma for astronomy for a long time”, says Rolf Güsten from the MPIfR, first author of the paper and Principal Investigator of the GREAT project until October 2018.
In the late 1970s, astro-chemical models suggested the possibility that HeH+ might exist at detectable abundances in local astrophysical nebulae, and would be most easily observed in so-called planetary nebula, ejected by sun-like stars in the last stage of their lifetime. The hard radiation field produced by the central white dwarf star with a temperature of more than 100,000 degrees drives ionization fronts into the ejected envelope, where HeH+ is predicted to form.
The molecule will emit its strongest spectral line at a characteristic wavelength of 0.149 mm (or a frequency of 2.01 terahertz). Unfortunately, Earth’ atmosphere is opaque at this wavelength for ground-based observatories, requiring this search to be performed from space or a high-flying observatory like SOFIA cruising above the absorbing layers of the lower atmosphere. “With recent advance¬ments in terahertz technologies is has now become possible to perform high-resolution spectroscopy at the required far-infrared wavelength”, explains Rolf Güsten. Operating the GREAT spectrometer aboard SOFIA the team now reports the unequivocal detection of HeH+ towards the envelope of the planetary nebula NGC 7027.
“The discovery of HeH+ is a dramatic and beautiful demonstration of Nature's tendency to form molecules", says David Neufeld from the Johns Hopkins University in Baltimore, a co-author of the paper. ”Despite the unpromising ingredients that are available, a mixture of hydrogen with the unreactive noble gas helium, and a harsh environment at thousands of degrees Celsius, a fragile molecule forms. Remarkably, this phenomenon can not only be observed by astronomers but also understood using theoretical models that we have developed.”
The detection of this special molecule brings a long search to a happy ending, and eliminates doubts that we might not understand the underlying formation and destruction as well as we thought. It is a reward for many years of instrument development work, and underlines the scientific value of airborne far-infrared astronomy.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!