Neutral Zinc-air battery with cathode NiCo/C-N shows outstanding performance
A team of researchers from the School of Chemistry and Chemical Engineering at Hunan University of Science and Technology have proposed a novel strategy for the synthesis of non-precious metal catalysts in zinc-air batteries that do not compromise its electroactivity, affordability and stability.
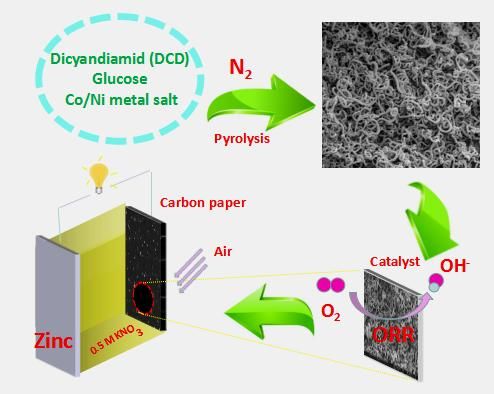
NiCo-doped C-N hollow nanotube composites prepared through direct pyrolysis of Ni/Co salt, dicyandiamide and glucose, present excellent performance as the cathode catalysts of neutral Zn-air battery.
Qingfeng Yi et al.
As a green and sustainable energy generator, zinc-air battery has attracted great attention from researchers due to its high specific energy, high current density, low cost, and environmental friendliness. Yet it is not without its drawbacks. The slow oxygen reduction reaction (ORR) of its cathode has become an obstacle to its commercial application. One possible solution is to use platinum (Pt) and Pt-based catalysts, but its high cost and scarce availability make it less ideal. In addition, alkaline KOH (or NaOH) is generally used as the electrolyte, but it leads to the generation of carbonates (CO32-) due to the dissolution of CO2 in the electrolyte as well as the spontaneous corrosion of the anodic zinc in strong alkaline media. This has the effect of slowing down the ionic conductivity of the electrolyte and battery life. Therefore, a neutral electrolyte should be used instead. To complement this, a non-precious metal ORR catalyst that can also perform in a neutral electrolyte needs to be found.
A team of researchers from the School of Chemistry and Chemical Engineering at Hunan University of Science and Technology introduced a novel strategy for the synthesis of non-precious metal catalysts with outstanding electroactivity for oxygen reduction reaction in neutral electrolyte. They prepared Ni/Co-doped C-N hollow nanotube composite catalysts (C-N, Co/C-N, Ni/C-N and Ni-Co/C-N) by a facile pyrolysis of Ni/Co salt, dicyandiamide and glucose. A corresponding zinc-air battery was assembled in a neutral medium using the prepared sample as the catalyst of the air electrode, showing superior discharge performance and stability.
The as-synthesized Ni/Co-doped C-N composites display a curly tubular structure, resulting in a tremendous active surface area and stability. A neutral zinc-air battery utilizing a 0.5 mol·L-1 KNO3 solution has been assembled by using the prepared composite catalyst coated on carbon paper as an air cathode, and Zn plate as an anode. The battery with the cathode catalyst Ni-Co/C-N delivers the open circuit voltage of 1.13 V and the maximum power density of 65 mW·cm-2. The constant discharge current density of 50, 100 and 150 mA·cm-2 can last 202, 93 and 11 hours respectively. A stable voltage plateau appears at various discharge current densities. The neutral zinc-air battery can be repeatedly discharged after replacing the zinc anode, showing the high stability of the cathodic catalyst and broad application prospects as a mobile power source.
Original publication
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.




























































