Simulating nature’s cosmic laboratory, one helium droplet at a time
Two astronomers from the Max Planck Institute for Astronomy and from the University of Jena have found an elegant new method to measure the energy of simple chemical reactions, under similar conditions as those encountered by atoms and molecules in the early solar system. Their method promises accurate measurements of reaction energies that can be used to understand chemical reactions under space conditions – including those reactions that were responsible of creating organic chemicals as the raw material for the development of life.
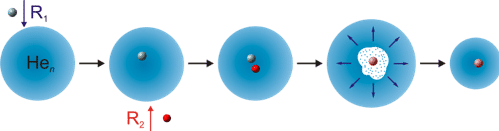
Schematic representation of the new method: Two reactants R1 and R2 are added to a helium droplet. The energy released in the resulting reaction decreases the droplet’s size. The decrease in size can be measured, and allows researchers to deduct the reaction energy.
Krasnokutskiy / MPIA
In order for life to form, nature needed plenty raw materials in the shape of complex organic molecules. Some of those molecules are likely to have formed long before, in space, during the birth of the Solar System. Systematic studies of the necessary chemical reactions, which take place on the craggy and convoluted surfaces of dust grains, were and are hampered by a lack of data. Which elementary reactions, involving which individual reactants are possible? What temperature is required for a reaction to take place? Which molecules are produced in those reactions? Now, Thomas Henning, director at the Max Planck Institute for Astronomy (MPIA), and Sergiy Krasnokutskiy of the MPIA’s Laboratory Astrophysics Group at the University of Jena have developed an elegant method to study such elementary surface reactions – using minute liquid helium droplets.
In the early solar system, long before the formation of Earth, complex chemical reaction took place, creating substantial amounts of organic molecules. The cosmic laboratory for these works of chemical synthesis was provided by grains of dust – clusters of mostly silicates and carbon, covered with a mantle of ice, with complicated and delicate tendrils and ramifications, and on this basis with one crucial property: A comparatively large surface on which chemical reactions could take place. In the millions of years that follows, many of those dust grains would cluster together for form ever larger structures, until finally, solid planets emerged, orbiting the young Sun.
Creating the raw ingredients for life
While all of the organic compounds synthesized on the grain surfaces would be destroyed by the unavoidable heat during planet formation, some of the molecules remained in waiting, encapsulated in, or clinging to the surface of, smallish grains or lumps of rock, as well as in the icy bodies of the comets. By one account of the history of life, once Earth’s surface had cooled sufficiently for liquid water to form, it was these grains and rocks, hitting Earth’s surface in the shape of meteorites, some of them landing in warm, small, ponds, that provided the chemical basis for life to form on our home planet.
In order to understand the early natural chemical experiments in our universe, we need to know the properties of the various reactions. For instance, do certain reactions need a specific activation energy to happen? What is the eventual product of a given reaction? Those parameters determine which reactions can happen under what conditions in the early Solar system, and they are key for any realistic reconstruction of early Solar system chemistry.
Scarce data about low-temperature surface reactions
Yet precise data on these reactions is surprisingly scarce. Instead, a substantial part of chemical research is dedicated to the study of such reactions in the gaseous phase, with the atoms and molecules floating freely, colliding, and forming compounds. But the crucial chemical reactions in space needed to build up larger organic molecules take place under markedly different conditions – on the surfaces of dust grains. This changes even the basic physics of the situation: When a new molecule is formed, the energy of the chemical bond formation is stored in the newly created molecule. If this energy is not passed on to the environment, the new molecule will quickly be destroyed. This prevents the formation of many species of in the gas phase. On a surface, or in a medium, where energy can readily be absorbed by the additional matter present, the conditions for certain types of reactions building complex molecules, step by step, are much more favorable.
Henning and Krasnokutskiy developed an elegant method for measuring the energetics of such reactions. Their mock-ups of cosmic laboratories are miniature helium droplets, a few nanometers in size, drifting in a high vacuum. The reactants – that is, the atoms or molecules meant to take part in the reaction – are brought into the vacuum chamber as gases, but in such minute amounts that helium droplets are overwhelmingly likely to pick up either a single molecule of each required species or none, but not more. The helium droplets act as a medium that, similar to the surface of a dust grain, can absorb reaction energy, allowing reactions to happen under similar conditions to those in the early Solar system. This reproduces a key feature of the relevant surface chemistry (although other properties, such as catalytic properties of a specific dust surface, are not modelled).
Nanodrops as measuring devices
Furthermore, the two astronomers used the helium nanodrops as energy measuring devices (calorimeters). As reaction energy is released into the drop, some of the Helium atoms will evaporate in a predictable fashion. The remaining drop is now smaller than before – a difference in size that can be measured using two alternative methods: an electron beam (a larger drop is easier to hit than a smaller one!) or a precise measurement of the pressure in the vacuum chamber created by Helium droplets hitting the wall, where larger droplets produce greater pressure. By calibrating their method using reactions that had been studied in detail beforehand, and whose properties are well-known, the two astronomers were able to increase the method’s accuracy considerably. All in all, the new method provides an elegant new way of investigating the formation pathway of complex organic molecules in space. This should enable researchers to be more specific about the raw materials nature had to work with in the run-up of the emergence of life on Earth. But there is more:
The first measurements using the new technique confirm a trend that had already been visible in other recent experiments: On surfaces, at low temperatures, carbon atoms are surprisingly reactive. The researchers found a surprisingly high number – almost a dozen – of reactions involving carbon atoms which are barrierless, that is, which do not require extra energy input to proceed, and hence can occur at very low temperatures. Evidently, the condensation of atomic gas at low temperatures is bound to lead to the formation of a large variety of organic molecules. But that large possible variety also means that molecules of each specific species will be very rare.
This, in turn, suggests that astronomers might be drastically underestimating the amount of organic molecules in outer space. When it comes to estimating abundances, astronomical observations examine the trace signatures (spectral lines) of each molecular species separately. If there are many different species of organic molecules out there, each separate species can “fly under the radar.” Its molecules might be present only in amounts too minute for astronomers to detect, and in addition, even the tell-tale signatures of the molecules (more generally those of specific functional groups common to different types of molecules) could be slightly altered, making the molecule evade detection. But added up, it is possible that all these separate species of molecule together could make up a substantial amount of matter in outer space – a hidden outer-space world of organic chemistry.