Duplicate or mirror? Laser light determines chirality of molecules
Seven of the ten most frequent medications contain chiral agents. These are molecules that occur in right- or left-handed forms. During chemical synthesis both forms usually occur in equal parts and have to be separated afterward, because chirality determines the agent’s effect in the body. Physicists at Goethe University have now succeeded in using laser light for the purpose of creating either right- or left-handed molecules.
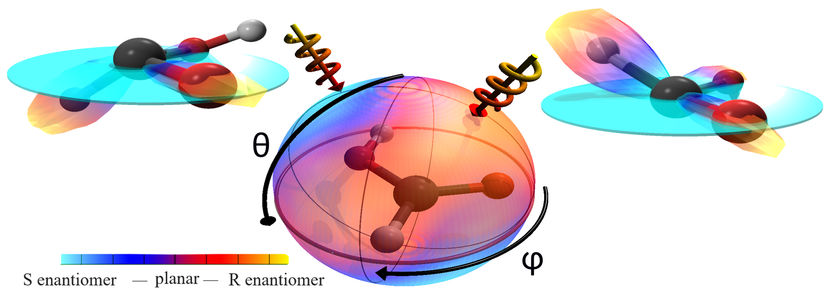
The formic acid model is in the centre. The color code of the surrounding sphere shows the direct chirality of the formic acid for every direction from which the laser comes. If the laser is directed from the right side (right arrow), it results in right-handed formic acid; if from the left, in left-handed formic acid. Both chiral formic acids reflect the common structure of the molecule.
Goethe-Universität Frankfurt am Main
“In pharmaceutics, being able to transition a molecule from one chirality to the other using light instead of wet chemistry would be a dream,” says Professor Reinhard Dörner from the Institute of Atomic Physics at Goethe University. His doctoral student Kilian Fehre has now brought this dream one step closer to coming true. His observation: the formation of the right- or left-handed version depends on the direction from which laser light hits the initiator.
For his experiment, Kilian Fehre used the planar formic acid molecule. He activated it with an intense, circularly polarized laser pulse to transition it to a chiral form. At the same time, the radiation caused the molecule to break into its atomic components. It was necessary to destroy the molecule for the experiment so that it could be determined whether a duplicate or mirror version was created.
Fehre used the “reaction microscope” (COLTRIMS method) that was developed at the Institute for Atomic Physics for the analysis. It allows the investigation of individual molecules in a molecular beam. After the molecule’s explosive breakdown, the data provided by the detector can be used to precisely calculate the direction and speed of the fragments’ paths. This makes it possible to reconstruct the molecule’s spatial structure.
In order to create chiral molecules with the desired chirality in the future, it has to be ensured that the molecules are oriented the same way with regard to the circularly polarized laser pulse. This could be achieved by orienting them beforehand using a long-wave laser light.
This discovery could also play a critical role in generating larger quantities of molecules with uniform chirality. However, the researchers believe that in such cases, liquids would probably be radiated rather than gases. “There is a lot of work to be done before we get that far,” Kilian Fehre believes.
The detection and manipulation of chiral molecules using light is the focus of a priority programme which goes by the memorable name “ELCH” and which has been funded by the German Research Council since 2018. Scientists from Kassel, Marburg, Hamburg and Frankfurt have joined forces in this programme. “The long-term funding and the close collaboration with the priority programme provide us with the necessary resources to learn to control chirality in a large class of molecules in the future,” concludes Markus Schöffler, one of the Frankfurt project managers of the priority programme.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.


























































