Zips on the nanoscale
New method of synthesising nanographene on metal oxide surfaces
nanostructures based on carbon are promising materials for nanoelectronics. However, to be suitable, they would often need to be formed on non-metallic surfaces, which has been a challenge – up to now. Researchers at FAU have found a method of forming nanographenes on metal oxide surfaces. Their research, conducted within the framework of collaborative research centre 953 – Synthetic Carbon Allotropes funded by the German Research Foundation (DFG), has now been published in the journal Science.
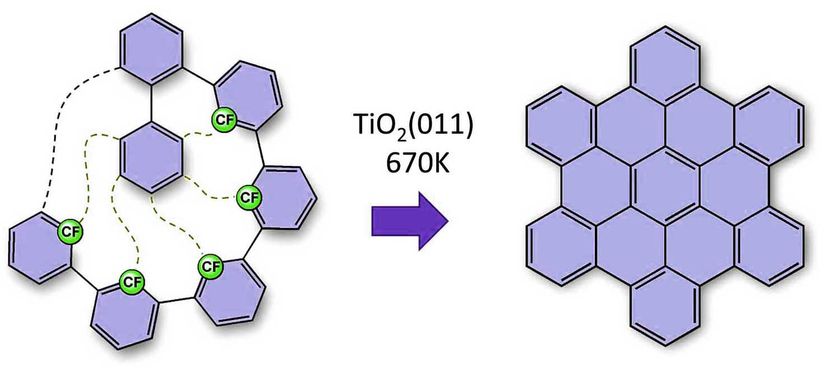
The desired nanographenes form like dominoes via cyclodehydrofluorination on the titanium oxide surface. All ‘missing’ carbon-carbon bonds are thus formed after each other in a formation that resembles a zip being closed.
FAU/Konstantin Amsharov
Two-dimensional, flexible, tear-resistant, lightweight, and versatile are all properties that apply to graphene, which is often described as a miracle material. In addition, this carbon-based nanostructure has unique electrical properties that make it attractive for nanoelectronic applications. Depending on its size and shape, nanographene can be conductive or semi-conductive – properties that are essential for use in nanotransistors. Thanks to its good electrical and thermal conductivity, it could also replace copper (which is conductive) and silicon (which is semi-conductive) in future nanoprocessors.
New: Nanographene on metal oxides
The problem: In order to create an electronic circuit, the molecules of nanographene must be synthesised and assembled directly on an insulating or semi-conductive surface. Although metal oxides are the best materials for this purpose, in contrast to metal surfaces, direct synthesis of nanographenes on metal oxide surfaces is not possible as they are considerably less chemically reactive. The researchers would have to carry out the process at high temperatures, which would lead to several uncontrollable secondary reactions. A team of scientists led by Dr. Konstantin Amsharov from the Chair of Organic Chemistry II have now developed a method of synthesising nanographenes on non-metallic surfaces, that is insulating surfaces or semi-conductors.
It’s all about the bond
The researchers’ method involves using a carbon fluorine bond, which is the strongest carbon bond. It is used to trigger a multilevel process. The desired nanographenes form like dominoes via cyclodehydrofluorination on the titanium oxide surface. All ‘missing’ carbon-carbon bonds are thus formed after each other in a formation that resembles a zip being closed. This enables the researchers to create nanographenes on titanium oxide, a semi-conductor. This method also allows them to define the shape of the nanographene by modifying the arrangement of the preliminary molecules. New carbon-carbon bonds and, ultimately, nanographenes form where the researchers place the fluourine atoms. For the first time, these research results demonstrate how carbon-based nanostructures can be manufactured by direct synthesis on the surfaces of technically-relevant semi-conducting or insulating surfaces. ‘This groundbreaking innovation offers effective and simple access to electronic nanocircuits that really work, which could scale down existing microelectronics to the nanometre scale,’ explains Dr. Amsharov.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.