New synthesis method for producing fluorinated piperidines
A team of chemists at the University of Münster led by Prof. Frank Glorius have developed a new, simple synthetic method for producing fluorinated piperidines –which had previously been very difficult. These compounds play a major role in the development of new active ingredients.
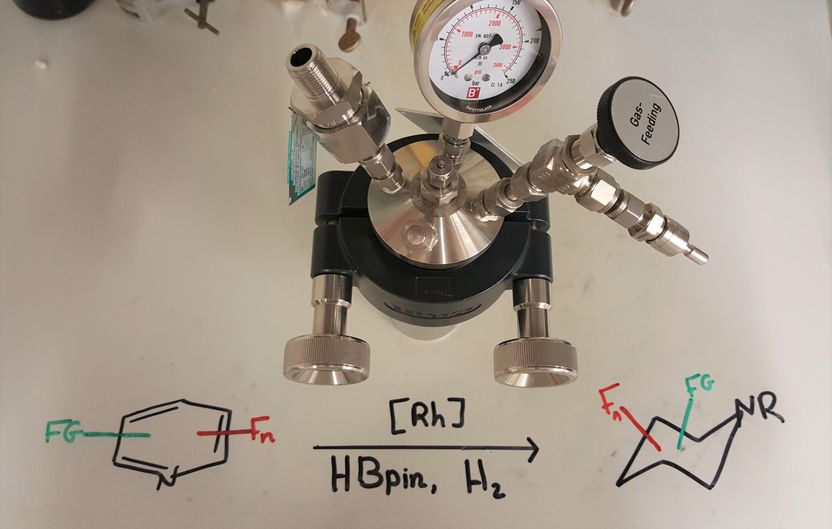
Pressure vessel (autoclave) for the hydrogenation of fluorinated pyridines. The reactions are carried out at a hydrogen pressure of 50 bar.
Copyright: Frank Glorius
Synthetic molecules are essential for many products in our lives: medicines, crop protection agents or special materials such as Teflon. These molecules have several components, which can be combined in a variety of ways, resulting in different properties. Both so-called piperidines and fluorinated groups are particularly important. Piperidines are small, ring-shaped chemical compounds. Since, as a result of their particular properties, fluorine atoms bring about dramatic changes in the properties of certain products –they are often integrated in pharmaceuticals. Hence around twenty percent of all medicines sold worldwide contain fluorine. Up to now, however, combining fluorine atoms and piperidines has always been an extremely laborious process. Now, for the first time, chemists at the University of Münster have developed a new, easy to do synthesis method for producing such fluorine-bearing piperidines. The study – written by Dr. Zackaria Nairoukh, Marco Wollenburg, Dr. Christoph Schlepphorst, Dr. Klaus Bergander and Prof. Frank Glorius – has just been published in the online edition of the Nature Chemistry journal.
In 2017, the Münster chemists had already published a study in journal “Science”, in which they presented a method by which cyclic, fluorinated molecules could be produced simply and quickly. The chemists’ new study follows on from this breakthrough. “Up to now, it had been very difficult to combine piperidines and fluorine molecules,” explains Prof. Frank Glorius from the Institute of Organic Chemistry at Münster University, “and this was despite the fact that together they have outstanding chemical properties relevant to the production of active ingredients.”
Methodology
The method developed by Frank Glorius and his team runs in two consecutive steps – but in the same vessel: the starting molecules used are easily accessible, fluorinated pyridines, so-called “aromatic” compounds. These compounds are flat and have a particularly high stability – which makes them inert for many chemical processes. In the method now published, the first step involves removing the aromaticity (“dearomatization”). This makes the second step possible, in which hydrogen atoms are transferred specifically to one side of the ring system (hydrogenation). The resulting fluorinated piperidines are now – in contrast to the aromatic starting substances – no longer flat, and this can be helpful for the formation of complex three-dimensional structures. For both reactions, the chemists use a catalyst. “Catalyst” is the term applied by chemists and biochemists to enzymes or other molecules that speed up the stages of a reaction or, indeed, make them possible at all.
The results can now be used to produce new types of valuable components, previously unavailable, for research in the fields of pharmaceuticals and agrochemicals. “We hope that these components can soon be produced in large quantities,” says Frank Glorius. The Münster University chemists want to use part of the grant of 2.5 million euros recently awarded by the European Research Council to work on a “renaissance” of the hydrogenation of aromatics, leading to the efficient production of such new types of molecule. “We’re going flat out in our efforts to try to understand more about the mechanism of catalysis, so that better catalysts can be developed and the potential of these reactions can be exploited to the full,” says Glorius.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.



























































