Molecular insights into spider silk
spider silk belongs to the toughest fibres in nature and has astounding properties. Scientists from the University of Würzburg discovered new molecular details of self-assembly of a spider silk fibre protein.
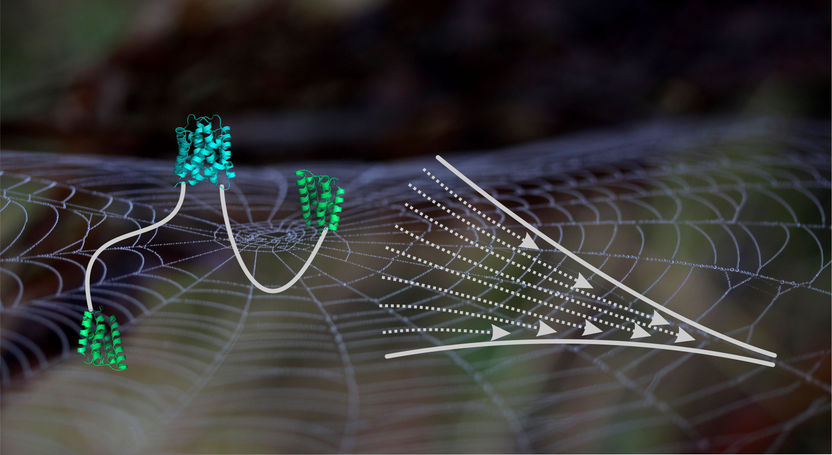
Schematic scheme of a spidroin consisting of an assembled C-terminal domain (cyan), the unfolded central domain (white line) and the N-terminal domains (green). Right hand side: scheme of a tapering spinning duct.
Hannes Neuweiler/Universität Würzburg
They are lightweight, almost invisible, highly extensible and strong, and of course biodegradable: the threads spiders use to build their webs. In fact, spider silk belongs to the toughest fibres in nature. Based on its low weight it even supersedes high-tech threads like Kevlar or Carbon. Its unique combination of strength and extensibility renders it in particular attractive for industry. Whether in aviation industry, textile industry, or medicine – potential applications of this magnificent material are manifold.
Since long time material scientists continue to try reproducing the fibre in the laboratory, but with limited success. Today, it is possible to manufacture artificial spider silk of similar properties as the prototype, but the molecular-level structural details responsible for material properties await to be disclosed. Now, scientists from the Julius-Maximilians-Universität Würzburg (JMU) delivered new insights. Dr Hannes Neuweiler, lecturer at the Institute of Biotechnology and Biophysics at the JMU, is in charge of this project.
A molecular clamp connects protein building blocks
“The silk fibres consist of protein building blocks, so-called spidroins, which are assembled by spiders within their spinning gland”, explains Neuweiler. The terminal ends of building blocks take special roles in this process. The two ends of a spidroin are terminated by an N- and a C-terminal domain.
The domains at both ends connect protein building blocks. In the present study, Neuweiler and colleagues took a close look at the C-terminal domain. The C-terminal domain connects two spidroins through formation of an intertwined structure that resembles a molecular clamp. Neuweiler describes the central result of the study: “We observed that the clamp self-assembles in two discrete steps. While the first step comprises association of two chain ends, the second step involves the folding of labile helices in the periphery of the domain”.
This two-step process of self-assembly was previously unknown and may contribute to extensibility of spider silk. It is known that stretching of spider silk is associated with unfolding of helix. Previous work, however, traced extensibility back to the unfolding of helices in the central segment of spidroins. “We propose that the C-terminal domain might also act as module that contributes to extensibility” explains Neuweiler.
Assisting material science
In their study Neuweiler and co-workers investigated protein building blocks of the nursery web spider Euprosthenops australis. They used genetic engineering to exchange individual moieties of building blocks and modified the protein chemically using fluorescent dyes. Finally, the interaction of light with soluble proteins disclosed that the domain assembles in two discrete steps.
Neuweiler describes the result as “a contribution to our molecular-level understanding of structure, assembly and mechanical properties of spider silk”. It may aid material scientists to reproduce natural spider silk in the laboratory. Currently, modified and synthetic spidroins are being used for this purpose. “Should the C-terminal domain contribute to flexibility of the thread, material scientists may modulate mechanical properties of the fibre through modulation of the C-terminal domain”, Neuweiler says.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
LANXESS to expand global capacity of high-performance rubber Nd-BR - Expansion at German, American and Brazilian sites
Checking people at airports with terahertz radiation - PTB succeeds in absolute measurement of terahertz radiation

Insights Into the Metabolism of Plastic-Eating Bacteria - Researchers have shown for the first time how bacteria break down common plastic coatings
Holistically sustainable alternative for plastics and bioplastics - Seed financing round for female-founded circular bioeconomy startup traceless materials