Simpler and safer method for handling a useful but foul-smelling gas in chemical synthesis
The chemical element sulfur is an important constituent in many pharmaceuticals and, consequently, it is desirable to be able to introduce sulfur-containing fragments efficiently in a broad range of chemical compounds. The Skrydstrup team provides an effective and safe way for introducing a small sulfur building block, which is generally difficult to work with, being a gas and with an extremely repulsive odor. Especially interesting in this work is that a gold-based catalyst is exploited for these specific reactions involving carbon-carbon double bonds.
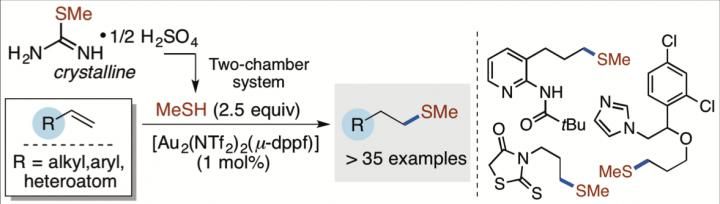
New method by Professor Troels Skrydstrup at Aarhus Universit for the hydrothiolation of π-systems with transition metal complexes.
Troels Skrydstrup
The research group focused on the use of the smallest carbon-containing thiol, namely methanethiol (MeSH). However, not only is MeSH the main compound responsible for bad breath and the smell of flatus, it is also highly flammable and therefore unsafe to work with in the laboratory. In the present study, researchers from iNANO and the Department of Chemistry, Aarhus University, report on how they successfully exploit their own invention, the two-chamber system , in order to avoid handling pressure cylinders with MeSH, and having to add the gas directly to the chemical reactions. The authors also demonstrate that a crystalline organic compound can be used to liberate an exact amount of MeSH upon activation in the two-chamber system.
In this work, the direct use of MeSH has been circumvented and a protocol for the delivery and use of a stoichiometric amount of gaseous MeSH has been developed without the need of pressure cylinders. The Skrydstrup group has demonstrated by the ex-situ generation of MeSH from a simple crystalline precursor in the two-chamber reactor that a gold(I)-mediated hydrothiolation of terminal alkenes is possible to provide the corresponding methyl sulfide in high yields. The reaction promoted by a gold(I) complex is also interesting as these complexes appear to operate as radical initiators from the mechanistic investigation undertaken.
Original publication
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.