A boost for biofuel cells
In chemistry, a reaction is spontaneous when it does not need the addition of an external energy input. How much energy is released in a reaction is dictated by the laws of thermodynamics. In the case of the spontaneous reactions that occur in the human body this is often not enough to power medical implants. Now, scientists at the Max Planck Institute for Intelligent Systems in Stuttgart, together with an international team of researchers, found a way to boost the energy output by storing and bundling the energy of many spontaneous enzyme reactions.
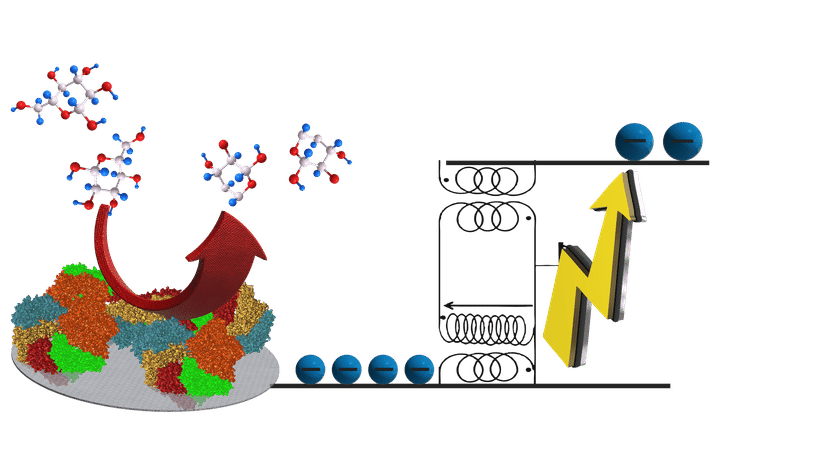
Boosting the energy output by storing and bundling the energy of many spontaneous enzyme reactions
Alejandro Posada
Chemical reactions in which electrons are released can be used to generate electricity. In order to power future medical implants, biofuel cells are being developed, where enzymes, derived from living organisms, are employed to liberate electrons, for instance during the oxidation of sugar molecules. An important general problem is that the energy and voltage of the electrons that can be generated in a biofuel cell is rather small. While it is easy in a household device to connect several batteries in series to boost the voltage, this is not possible in the human body, as each biofuel cell is necessarily immersed in the same fluid at the same electrical potential so that voltages cannot be added up. This means that biofuel cells can presently in most of the cases only generate much less than 1 Volt, which severely limits practical applications.
This is not only a matter of engineering, but the fundamental laws of thermodynamics also put limitations on the energy that can be released in the enzymatic reactions. The challenge is therefore to develop new approaches for biofuel cells that can circumvent these constraints.
Researchers from the University of Bordeaux, the French CNRS, the University of Stuttgart and the Max Planck Institute for Intelligent Systems (MPI-IS) have now found a clever way to overcome the restrictions imposed by thermodynamics. They managed to temporarily store the electrons released when glucose is oxidized and to use some of this energy to autonomously boost the voltage of the remaining electrons. “No outside power is needed”, says Emmanuel Suraniti, postdoctoral fellow at the MPI-IS and first author of the study that is published in Nature Communications.
The principle of operation is very general and relies in storing the energy of the electrons temporarily in an electromagnetic field. To achieve this, a small electronic circuit – powered by the chemical reaction itself – is integrated into the biofuel cell in order to harvest the electricity and boost the voltage. Thus, the concept makes it possible to turn simple biomolecules into high-energy fuels.
“Converting the starting compounds into electrons of high energy via the biofuel cell opens up completely new possibilities to power electronics and to synthesize important chemicals”, says Peer Fischer, who heads the Micro, Nano and Molecular Systems lab at the Max Planck Institute for Intelligent Systems and who is a Professor of Physical Chemistry at the University of Stuttgart. “It is now possible to use the oxidation of abundant low-energy molecules to synthesize precious molecules that need higher energies to form, which is a completely new concept.”
Apart from biofuel cells that generate useful voltages, the researchers envision reactions inside the body where the oxidation of glucose drives the synthesis of large drug molecules – something that thermodynamics would otherwise not allow, but which becomes possible in what may well be a first demonstration of the direct integration of electronics during a chemical reaction.
Original publication
Most read news
Original publication
“Uphill production of dihydrogen by enzymatic oxidation of glucose without an external energy source”; Emmanuel Suraniti, Pascal Merzeau, Jérôme Roche, Sébastien Gounel, Andrew G. Mark, Peer Fischer, Nicolas Mano, Alexander Kuhn; Nature Communications; 9, 3229 (2018).
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.