Insight into catalysis through novel study of X-ray absorption spectroscopy
An international team has made a breakthrough at BESSY II. For the first time, they succeeded in investigating electronic states of a transition metal in detail and drawing reliable conclusions on their catalytic effect from the data. These results are helpful for the development of future applications of catalytic transition-metal systems.
HZB

Manganese compounds also play a role as catalysts in photosynthesis.
HZB

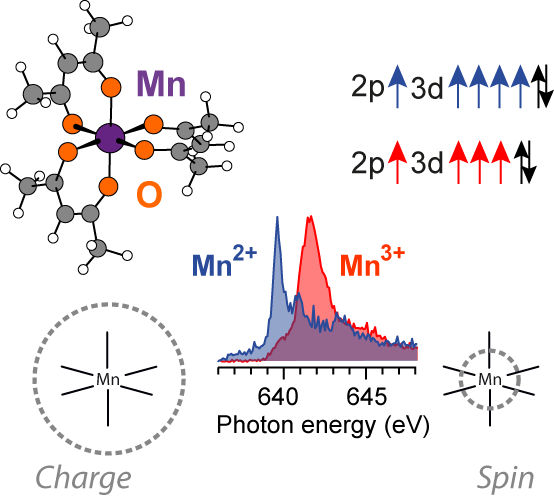
Many important processes in nature depend on catalysts, which are atoms or molecules that facilitate a reaction, but emerge from it themselves unchanged. One example is photosynthesis in plants, which is only possible with the help of a protein complex comprising four manganese atom sites at its centre. Redox reactions, as they are referred to, often play a pivotal role in these types of processes. The reactants are reduced through uptake of electrons, or oxidized through their release. Catalytic redox processes in nature and industry often only succeed thanks to suitable catalysts, where transition metals supply an important function.
Soft x-rays at BESSY II
These transition metals and in particular their redox or oxidation state can be examined particularly well using soft X-rays, because electronic states can be precisely measured using X-ray spectroscopy. In what is known as L-edge absorption spectroscopy, electrons from the 2p shell of the transition metal are excited so that they occupy free d-orbitals. An energy difference can be determined from the X-ray absorption spectrum that reflects the oxidation state of the molecule or the catalyst in a known way. However, exactly where the electrons are absorbed or released by the catalyst during a redox reaction, i.e. exactly how the charge density in the catalyst varies with oxidation state, was previously difficult to verify. This was mainly due to the lack of reliable methods for the theoretical description of charge densities in catalyst molecules in ground and excited states, and to the difficulty in obtaining reliable experimental data. If the transition metals are located in larger complex organic molecule complexes, as they typically are for real redox catalysts, their study becomes extremely difficult because the X-rays lead to damage in the sample.
Sample in solution examined in different oxidation states
Now for the first time, an international team from the Helmholtz-Zentrum Berlin, Uppsala University (Sweden), Lawrence Berkeley National Laboratory in Berkeley (USA), Manchester University (Great Britain), and the SLAC National Accelerator Laboratory at Stanford University (USA) has succeeded in studying manganese atoms in different oxidation states – i.e. during different stages of oxidation – in various compounds through in operando measurements at BESSY II. To accomplish this, Philippe Wernet and his team introduced the samples into various solvents, examined jets of these liquids using X-rays, and compared their data against novel calculations from Marcus Lundberg's group at Uppsala University. “We succeeded in determining how – and above all why – the X-ray absorption spectra shift with the oxidation states”, says theoretician Marcus Lundberg. PhD students Markus Kubin (HZB) with his experimental expertise and Meiyuan Guo (Uppsala University) with his theoretical expertise reflect the interdisciplinary approach of the study and they contributed equally as first authors of the paper.
Breakthrough through a combination of theory and experiment
“We combined a novel experimental setup with quantum chemical calculations. In our opinion, we have achieved a breakthrough in the understanding of organometallic catalysts”, says Wernet. “For the first time, we were able to empirically test and validate calculations for oxidation and reduction that do not take place locally on the metal, but instead on the entire molecule.” “These findings are a cornerstone for future work in more complex systems, like the tetra manganese cluster in photosynthesis. They will facilitate new understanding of redox processes for the manganese catalyst in the Photosystem II protein complex”, says Junko Yano, Senior Scientist of Molecular Biophysics and Integrated Bioimaging Division (MBIB) and the Joint Center for Artificial Photosynthesis (JCAP) at Lawrence Berkeley National Laboratory, who is conducting detailed research of photosynthesis.
Original publication
Markus Kubin, Meiyuan Guo, Thomas Kroll, Heike Löchel, Erik Källman, Michael L. Baker, Rolf Mitzner, Sheraz Gul, Jan Kern, Alexander Föhlisch, Alexei Erko, Uwe Bergmann, Vittal Yachandra, Junko Yano, Marcus Lundberg and Philippe Wernet; "Probing the oxidation state of transition metal complexes: a case study on how charge and spin densities determine Mn L-edge X-ray absorption energies"; Chemical Science; 2018
Original publication
Markus Kubin, Meiyuan Guo, Thomas Kroll, Heike Löchel, Erik Källman, Michael L. Baker, Rolf Mitzner, Sheraz Gul, Jan Kern, Alexander Föhlisch, Alexei Erko, Uwe Bergmann, Vittal Yachandra, Junko Yano, Marcus Lundberg and Philippe Wernet; "Probing the oxidation state of transition metal complexes: a case study on how charge and spin densities determine Mn L-edge X-ray absorption energies"; Chemical Science; 2018
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.



























































