Efficient synthesis of multi-substituted anilines by domino rearrangement
Advertisement
anilines have been widely used in medicine, in particular in acetaminophen pain killers. They are also used in organic materials, such as liquid crystals and organic light-emitting diodes. The ability to efficiently synthesize a new class of aniline derivatives will enable further developments in medical and materials science.
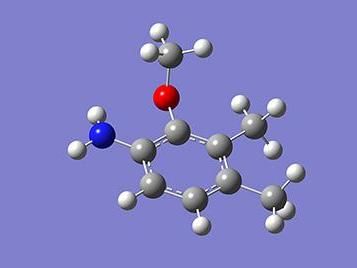
These are multiply-substituted anilines.
Itaru Nakamura
In a recent study, Yasuhiro Ishida, Itaru Nakamura, and Masahiro Terada at Tohoku University have reported that anilines, in which more than two substituents were placed sequentially, were synthesized from readily accessible substrates (ortho-alkylated N-methoxyanilines) in a selective manner.
Until now, preparation of multiply substituted anilines by conventional synthetic methods has been inefficient due to low selectivity.
Researchers found the reaction in the current study was effectively promoted by cationic copper catalysts under mild reaction conditions.
Substrates which have a substituent at the para position were efficiently converted to the desired anilines in which the four substituent are sequentially arranged, in good yields. Since conventional methods for synthesizing multi-substituted anilines have required multiple steps and often suffered from low efficiency, the new method offers vast improvement.
Mechanistic studies suggest that the unique transformation is regarded as "domino rearrangement," in which the first [1,3] rearrangement of the methoxy group induces the second [1,2] rearrangement from the ortho position to the meta position.
It is expected that the products obtained by the new method will contribute to the development of medicine and organic materials. Further development of the catalytic domino rearrangement reaction will lead to fewer processes and selective synthesis of a wide variety of multi-substituted aromatic compounds.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.