Double graphene sandwich provides protection against corrosion
Advertisement
”Normally it’s not a huge success that not a damn thing has happened after 120 days.”
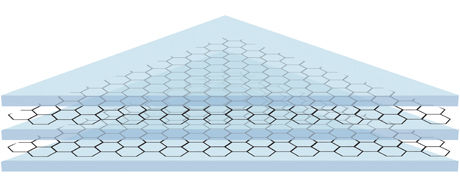
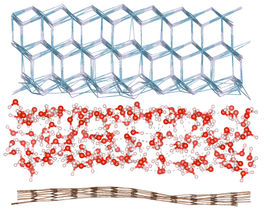
Like the layered structure of a sandwich, graphene sheets (which are excellent barriers) can be layered with polymers to make a coating that is both strong, durable and exceptionally good at preventing corrosion
Technical University of Denmark
These words belong to Professor Peter Bøggild after a four-month period where a novel type of anticorrosion coating was tested. The nanomaterial graphene plays a crucial role in this new type of coating. Furthermore, the result is a response to a line of studies showing graphene only provides a very short-term protection against corrosion, and actually makes it worse in the long run. When the article “Graphene as a Long-Term Metal Oxidation Barrier: Worse Than Nothing” was published, Peter Bøggild’s team was close to giving up. Now, five years later, a bright PhD student discovered how to solve the problem and the answer is… a double sandwich.
Since the 1930’s, zinc has been a key component in counteracting corrosion, because zinc coating creates a galvanic barrier. In simple terms, the elements, wind, and water will start eroding the zinc in the coating before it reaches the steel under the coating. For a long time however, different and better solutions to protect metal against corrosion have been sought.
The obvious answer was graphene. The single-layer of carbon atoms – the wonder material - is strong, stable and impermeable, and should be perfect for corrosion protection.
“Unfortunately, it has turned out that coating metal with graphene is a bad idea, because the high conductivity and “nobility” create galvanic corrosion. Zinc “sacrifices” itself so the metal stays unharmed, whereas graphene is the stronger player in relation to steel and thereby forces the metal to dissolve. If the graphene was 100% perfect without any flaws or holes, it would be fine, but the reality is rarely so. Even the smallest crack in the graphene would instantly accelerate an aggressive flow of metal ions away from the metal,” Peter Bøggild explains. But then it happened nonetheless. “Now, we have finally made graphene work as a coating to protect against corrosion”, Peter Bøggild says.
Initially one layer, and then another one
Peter Bøggild’s PhD student, Feng Yu, came up with the idea of a sandwich structure, where a thin layer of polymer separates the graphene electrically from the surface. The coating was tested on AA (aircraft aluminum), which was submerged in simulated seawater, a hostile environment for most metals. The researchers performed potentio-dynamic measurements and used impedance spectroscopy to check on the condition of the coating, and to measure the flow of corrosion after 1 day, 30 days and 120 days. The coating was more effective than before, but still was not durable.
“During the first month the corrosion indicators started showing on the aluminum, and we could see that the corrosion current increased significantly”, Peter Bøggild says.
Then Feng Yu came up with the double sandwich, where an extra layer of polymer and graphene gives the following not easily pronounced sequence: polymer-graphene-polymer- graphene -polymer (PGPGP).
This approach turned out to be not just twice as good, but actually performed a thousand times better. “After four months we concluded that the coating was unaltered, and the corrosion current was just as ridiculously low as when we started, as if the metal had not realised that it had been submerged in salt water for four months”, Peter Bøggild says.
Peter Bøggild also has an explanation as to why it works: There will always be holes and cracks in a single layer of graphene. After all, it is only one atom in thickness. After some time the galvanic corrosion of the metal will start where the graphene doesn’t cover. But with two layers everything is different. The chance that there is a hole in the graphene in the exact same spot is very unlikely. It is like the difference between painting a wall once or twice, it makes a major difference in getting rid of the dry spots.
Every imaginable possibility
Over the last two years, Feng Yu has compared every imaginable combination of polymer and graphene: 3 polymer layers without graphene, 1 layer of graphene, 2 layers of graphene, 3 layers of graphene, graphene layers directly upon each other, graphene layers separated by polymer, graphene-oxide etc. 1 layer is not enough, and 3 layers do not improve the protection compared to 2 layers.
The conclusion is clear: A double sandwich solves the problem.