Nanocrystalline diamonds used to study materials under extreme conditions
Using a nanocrystalline diamond built by plasma vapor deposition, Yogesh Vohra, Ph.D., has already produced a pressure nearly two times greater than that found at the center of the Earth.

Single-crystal gem diamond sits on microscope under laser light.
UAB
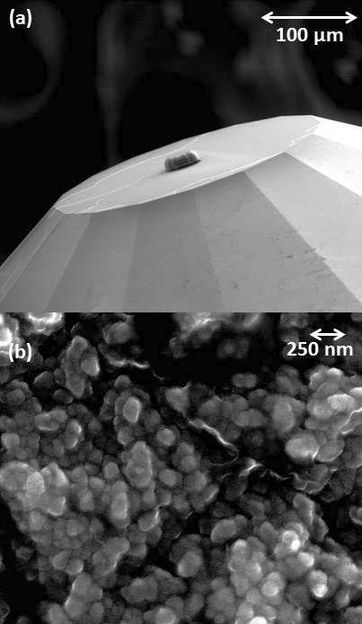
This nubbin on the flat surface of a gem diamond (top) is a nanocrystalline diamond, half the width of an average human hair. Higher magnification (bottom) shows the granular structure of the nanocrystalline diamond.
UAB


Now he reports that the manufacturing process of these novel, nanocrystalline-diamond micro-anvils has proved to be "remarkably consistent" and demonstrates "a high level of reproducibility in fabrication."
These results are encouraging for continued research to study materials under extreme conditions of pressure and temperature, says Vohra, a professor and university scholar of physics in the UAB College of Arts and Sciences at the University of Alabama at Birmingham.
The nanocrystalline diamond looks like a tiny nubbin of material grown on top of the flat culet surface of a one-third-carat gem diamond. To construct the nubbin, the gem diamond is coated with a tungsten thin film that has a 15- to 20-micrometer circle etched out at the center. The nanocrystalline diamond begins to grow as tiny diamond grains in that circle on top of the gem diamond surface. The grains form through vapor deposition from plasma made by heating methane, hydrogen and nitrogen gases.
Plasma is a hot, ionized gaseous substance that is the fourth state of matter after liquids, solids and gasses. Grains of nanocrystalline diamond are typically between 5 and 100 nanometers in size.
Vohra and UAB colleagues looked at early-stage nucleation morphologies of the nanocrystals at one, three and 15 minutes after start of synthesis. They found that nucleation of nanocrystalline diamonds begins rapidly, and without need of pre-growth surface seeding with tiny specks of diamond. In contrast, such a seeding is required for diamond growth on some other surfaces.
After only one minute of growth, electron microscope images showed substantial nucleation sites on the surface of the single-crystal gem diamond anvil. At three minutes, only small areas of the gem surface lacked nanocrystalline diamond coverage, and by 15 minutes, there was complete and uniform coverage by nanocrystalline grains that begin to clump together over the entire growth region.
Growth slowed between three and six hours, and the nanocrystalline diamond tended to coalesce into a hemi-spherical structure. Vohra says this geometry has been observed consistently over every two-stage growth experiment the UAB researchers have performed. In addition, there seems to be a geometrical limit to the total dimensions of growth.
The nanocrystalline nubbin greatly enhances the pressures achievable with diamond micro-anvils. Single-crystal gem diamonds with a culet size of 300 microns, without the nanocrystalline nubbin, can generate only 75 gigapascals of pressure. When the nanocrystalline diamond is added, the micro-anvils can generate as much as 500 gigapascals of pressure. The UAB researchers hope to reach a pressure of 1,000 gigapascals, or one terapascal, of pressure with their nanocrystalline diamond micro-anvils. That is close to the pressure at the center of the planet Saturn.
This immense pressure can potentially create as yet unknown new materials and are also utilized to study phase changes and compression behavior of materials. In the natural world, such immense forces deep underground can turn carbon into diamonds, or volcanic ash into slate.
The UAB team also examined nanocrystalline diamond micro-anvils that showed detachment during the compression and decompression in a diamond-anvil cell device. Using electron force microscopy, scanning electron microscopy and Raman spectroscopy, the researchers found that the detachment failure occurred in the bulk of the single-crystal gem diamond anvil below the culet surface, not at the interface between the gem diamond and the nanocrystalline diamond nubbin.
This indicated that the interfacial adhesive strength between the gem diamond and the nanocrystalline diamond nubbin appears to be substantial, and that the interface can survive ultrahigh shear stresses.
Vohra says UAB researchers will continue studies to manipulate grain size and the adhesive strength at the interface to optimize nanocrystalline diamond micro-anvils for high-pressure research.
Original publication
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.