Bacteria produce gold by digesting toxic metals
High concentrations of heavy metals, like copper and gold, are toxic for most living creatures. This is not the case for the bacterium C. metallidurans, which has found a way to extract valuable trace elements from a compound of heavy metals without poisoning itself. One interesting side-effect: the formation of tiny gold nuggets. A team of researchers from Martin Luther University Halle-Wittenberg (MLU), the Technical University of Munich (TUM) and the University of Adelaide in Australia has discovered the molecular processes that take place inside the bacteria.

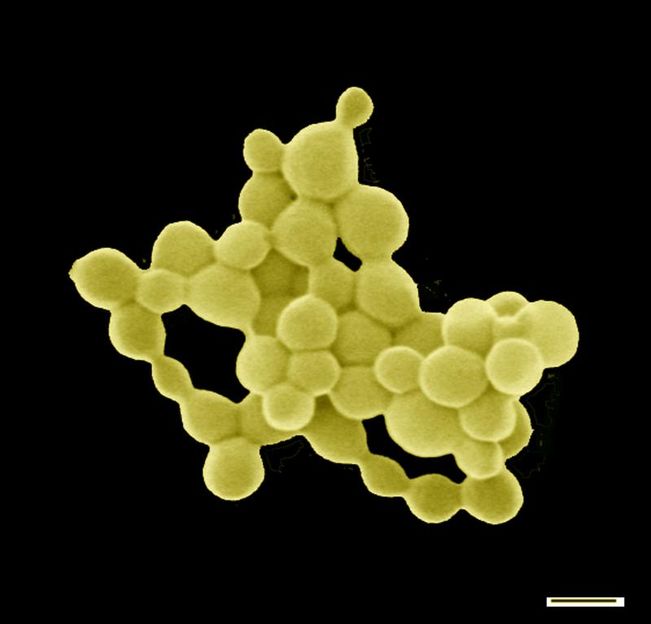
C. metallidurans can produce small gold nuggets
American Society for Microbiology
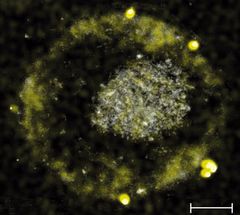
Another image of the tiny gold nuggets
Technische Universität München
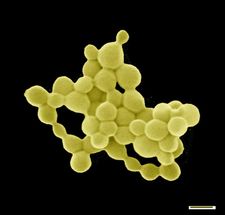
The rod-shaped bacterium C. metallidurans primarily lives in soils that are enriched with numerous heavy metals. Over time some minerals break down in the soil and release toxic heavy metals and hydrogen into their environment. "Apart from the toxic heavy metals, living conditions in these soils are not bad. There is enough hydrogen to conserve energy and nearly no competition. If an organism chooses to survive here, it has to find a way to protect itself from these toxic substances," explains Professor Dietrich H. Nies, a microbiologist at MLU. Together with his Australian counterpart, Professor Frank Reith from the University of Adelaide, he was able to prove in 2009 that C. metallidurans is able to deposit gold biologically. Why it does this and the exact processes that take place remained unknown. Now the researchers have finally been able to solve the mystery.
Gold enters the bacteria the same way as copper. Copper is a vital trace element for C. metallidurans however it is toxic in large quantities. When the copper and gold particles come into contact with the bacteria, a range of chemical processes occur: Copper, which usually occurs in a form that is difficult to be taken up, is converted to a form that is considerably easier for the bacterium to import and thus is able to reach the interior of the cell. The same also happens to the gold compounds.
When too much copper has accumulated inside the bacteria, it is normally pumped out by the enzyme CupA. "However, when gold compounds are also present, the enzyme is supressed and the toxic copper and gold compounds remain inside the cell. Copper and gold combined are actually more toxic than when they appear on their own," says Dietrich H. Nies. To solve this problem, the bacteria activate another enzyme - CopA. This enzyme transforms the copper and gold compounds into their originally difficult to absorb forms. "This assures that fewer copper and gold compounds enter the cellular interior. The bacterium is poisoned less and the enzyme that pumps out the copper can dispose of the excess copper unimpeded. Another consequence: the gold compounds that are difficult to absorb transform in the outer area of the cell into harmless gold nuggets only a few nanometres in size," says Nies.
In nature, C. metallidurans plays a key role in the formation of so-called secondary gold, which emerges following the breakdown of primary, geologically created, ancient gold ores. It transforms the toxic gold particles formed by the weathering process into harmless gold particles, thereby producing gold nuggets.
The study conducted by the joint German-Australian research team provides important insights into the second half of the bio-geochemical gold cycle. Here primary gold metal is transformed by other bacteria into mobile, toxic gold compounds, which is transformed back into secondary metallic gold in the second half of the cycle. Once the entire cycle is understood, gold can also be produced from ores containing only a small percentage of gold without requiring toxic mercury bonds as was previously the case.
Original publication
Nicole Wiesemann, Lucy Bütof, Martin Herzberg, Gerd Hause, Lutz Berthold, Barbara Etschmann, Joël Brugger, Gema Martinez-Criado, Dirk Dobritzsch, Sacha Baginsky, Frank Reith, and Dietrich H. Nies; "Synergistic Toxicity of Copper and Gold Compounds in Cupriavidus metallidurans"; Appl. Environ. Microbiol.; 2017
Bütof, L., N. Wiesemann, M. Herzberg, M. Altzschner, A. Holleitner, F. Reith and D. H. Nies; "Synergetic gold-copper detoxification at the core of gold biomineralisation in Cupriavidus metallidurans." Metallomics; 2018
Original publication
Nicole Wiesemann, Lucy Bütof, Martin Herzberg, Gerd Hause, Lutz Berthold, Barbara Etschmann, Joël Brugger, Gema Martinez-Criado, Dirk Dobritzsch, Sacha Baginsky, Frank Reith, and Dietrich H. Nies; "Synergistic Toxicity of Copper and Gold Compounds in Cupriavidus metallidurans"; Appl. Environ. Microbiol.; 2017
Bütof, L., N. Wiesemann, M. Herzberg, M. Altzschner, A. Holleitner, F. Reith and D. H. Nies; "Synergetic gold-copper detoxification at the core of gold biomineralisation in Cupriavidus metallidurans." Metallomics; 2018
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

SensorData Instruments e.K. - Lüdenscheid, Germany

Stora Enso enters into strategic partnership with Swedish University of Agricultural Sciences
Robert_S._Mulliken
Category:Biofuel_power_stations_by_country

Stronger paper bags, reused repeatedly then recycled for biofuel could be future

TBFA GMBH E. Lampe & TBAG Schweiz - Bad Birnbach, Germany

The role of hydrophobic molecules in catalytic reactions - Optimising electrochemical processes is one of the challenges in developing technologies for renewable energies. New research findings could provide assistance here.