UV laser photolyses to enhance diamond growth
Energy influences the rates of chemical reactions dramatically. Simply heating a gas-phase reaction system deposits energy indiscriminately in internal and translational motions of precursor and intermediate molecules. More specific excitations of energy states in the molecules can control the courses of a reaction. Control of chemical reactions is an intriguing concept. The practical reasons for seeking such a control ranging from suppressing unwanted side products to synthesizing new material structures. With the significant advances in laser technology, lasers can now provide unique means of selectively driving chemical reactions by exciting specific transitions in reactant molecules. Ultraviolet photochemistry has long been exploited to gain chemical control in molecular reactions motivated by suppressing side product channels to obtain the desired deposit. However, there have been few successes in practical material synthesis because the photochemical effects have been believed to be too weak. Nevertheless, selectivity among various competing chemical processes in material synthesis is attractive because it enables a better understanding of the reacting channels, leading to process control and improvements.
Researchers at the University of Nebraska-Lincoln, USA, reported on a new laser-enabled synthesis route to explore the advantages of laser photochemistry in practical material synthesis in a recent article in Light: Science & Applications. In this work, it is demonstrated that UV laser photolysis of hydrocarbon species altered the flame chemistry to promote the diamond growth rate and film quality. The authors found that the UV laser photolysis plays a key role in suppressing the formation of the side products, nondiamond carbons. This discovery suggests the great potential of the laser photolysis forsignificantly improving the synthesis of a broad range of technically important materials.
Original publication
Lisha Fan, Loic Constantin, Dawei Li, Lei Liu, Kamran Keramatnejad, Clio Azina, Xi Huang, Hossein Rabiee Golgir, Yao Lu, Zahra Ahmadi, Fei Wang, Jeffrey Shield, Bai Cui, Jean-Francois Silvain and Yong Feng Lu; "Ultraviolet laser photolysis of hydrocarbons for nondiamond carbon suppression in chemical vapor deposition of diamond films"; Light: Science & Applications; 2018
Most read news
Original publication
Lisha Fan, Loic Constantin, Dawei Li, Lei Liu, Kamran Keramatnejad, Clio Azina, Xi Huang, Hossein Rabiee Golgir, Yao Lu, Zahra Ahmadi, Fei Wang, Jeffrey Shield, Bai Cui, Jean-Francois Silvain and Yong Feng Lu; "Ultraviolet laser photolysis of hydrocarbons for nondiamond carbon suppression in chemical vapor deposition of diamond films"; Light: Science & Applications; 2018
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.
Last viewed contents
Calibre acquires RheinPerChemie GmbH from Evonik
AkzoNobel expands Performance Coatings research facility in Houston

Beilstein-Institut zur Förderung der Chemischen Wissenschaften - Frankfurt am Main, Germany
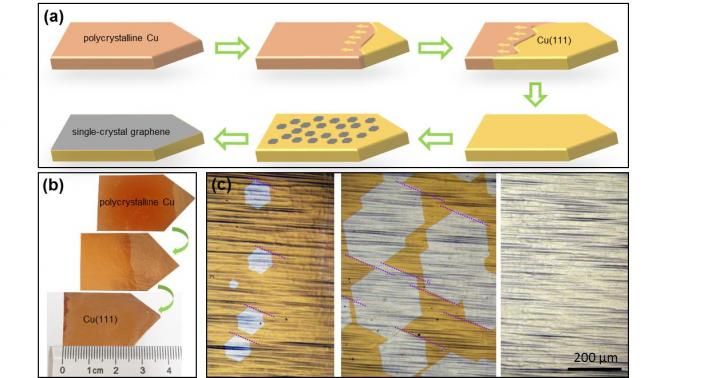
Large single-crystal graphene is possible! - The target of large, cheap and quick graphene synthesis achieved: 5 x 50 cm2 and beyond
More Economical and Sustainable Rechargeable Batteries - Ultralow-concentration electrolyte for lithium-ion batteries
The First Digital Humidity/Temperature Sensor!

WITec announces Paper Award 2022 winners - The prizes go to scientists from the US, Germany and China
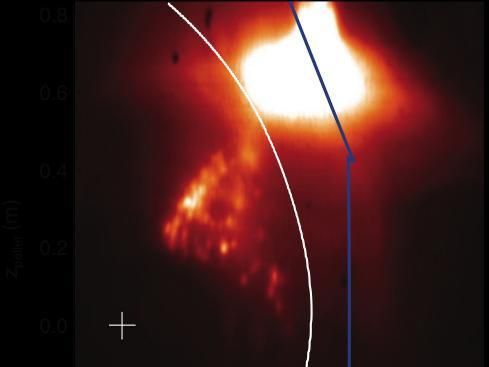
Mixing an icy cocktail to safely cool hot plasma - A promising solution to controlling hot plasmas in fusion devices