Coupling experiments to theory to build a better battery
Lithium-sulfur batteries are promising candidates for replacing common lithium-ion batteries in electric vehicles since they are cheaper, weigh less, and can store nearly double the energy for the same mass. However, lithium-sulfur batteries become unstable over time, and their electrodes deteriorate, limiting widespread adoption.
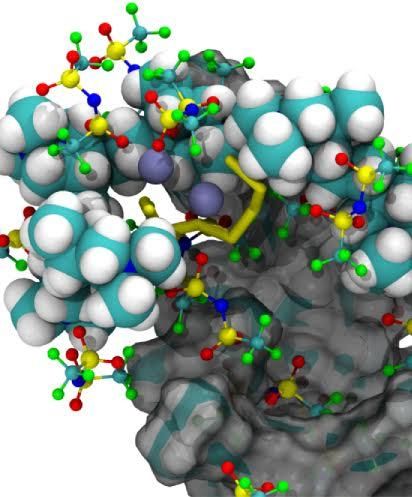
This illustration shows the formation of complex ion clusters during the cycling of a lithium-sulfur battery cell. The clusters consist of cationic polymer binders, battery electrolyte, and anionic sulfur-active materials.
Berkeley Lab
Now, a team of researchers led by scientists at the U.S. Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) have reported that a new lithium-sulfur battery component allows a doubling in capacity compared to a conventional lithium-sulfur battery, even after more than 100 charge cycles at high current densities, which are key performance metrics for their adoption in electric vehicles (EVs) and in aviation.
They did it by designing a new polymer binder that actively regulates key ion transport processes within a lithium-sulfur battery, and have also shown how it functions on a molecular level.
"The new polymer acts as a wall," said Brett Helms, a staff scientist at Berkeley Lab's Molecular Foundry and corresponding author of the study. "The sulfur is loaded into the pores of a carbon host, which are then sealed by our polymer. As sulfur participates in the battery's chemical reactions, the polymer prevents the negatively charged sulfur compounds from wandering out. The battery has great promise for enabling the next generation of EVs."
When a lithium-sulfur battery stores and releases energy, the chemical reaction produces mobile molecules of sulfur that become disconnected from the electrode, causing it to degrade and ultimately lowering the battery's capacity over time. To make these batteries more stable, researchers have traditionally worked to develop protective coatings for their electrodes, and to develop new polymer binders that act as the glue holding battery components together. These binders are intended to control or mitigate the electrode's swelling and cracking.
The new binder goes a step further. Researchers from the Organic Synthesis Facility at Berkeley Lab's Molecular Foundry, a research center specializing in nanoscale science, designed a polymer to keep the sulfur in close proximity to the electrode by selectively binding the sulfur molecules, counteracting its migratory tendencies.
The next step was to understand the dynamic structural changes that are likely to occur during charging and discharging as well as at different states of charge. David Prendergast, who directs the Foundry's Theory Facility, and Tod Pascal, a project scientist in the Theory Facility, built a simulation to test the researchers' hypotheses about the polymer's behavior.
"We can now reliably and efficiently model sulfur chemistry within these binders based on learning from detailed quantum mechanical simulations of the dissolved sulfur-containing products," stated Prendergast.
Their large-scale molecular dynamics simulations, conducted on supercomputing resources at Berkeley Lab's National Energy Research Scientific Computing Center (NERSC), confirmed that the polymer has an affinity for binding the mobile sulfur molecules, and also predicted that the polymer would likely show a preference for binding different sulfur species at different states of charge for the battery. Experiments conducted at Berkeley Lab's Advanced Light Source and Argonne National Laboratory's Electrochemistry Discovery Lab confirmed these predictions.
The research team took their study one step further by also examining the performance of lithium-sulfur cells made with the new polymer binder. Through a set of experiments, they were able to analyze and quantify how the polymer affects the chemical reaction rate in the sulfur cathode, which is key to achieving high current density and high power with these cells.
By nearly doubling the battery's electrical capacity over long-term cycling, the new polymer raises the bar on the capacity and power of lithium-sulfur batteries.
The combined understanding of the synthesis, theory, and characteristics of the new polymer have made it a key component in the prototype lithium-sulfur cell at DOE's Joint Center for Energy Storage Research (JCESR).
Original publication
Most read news
Original publication
Longjun Li, Tod A. Pascal, Justin G. Connell, Frank Y. Fan, Stephen M. Meckler, Lin Ma, Yet-Ming Chiang, David Prendergast & Brett A. Helms; "Molecular understanding of polyelectrolyte binders that actively regulate ion transport in sulfur cathodes"; Nature Comm.; 2017
Topics
Organizations
Other news from the department science
These products might interest you
OCA 200 by DataPhysics
Using contact angle meter to comprehensively characterise wetting behaviour, solids, and liquids
With its intuitive software and as a modular system, the OCA 200 answers to all customers’ needs
Tailor-made products for specific applications by IPC Process Center
Granulates and pellets - we develop and manufacture the perfect solution for you
Agglomeration of powders, pelletising of powders and fluids, coating with melts and polymers
Dursan by SilcoTek
Innovative coating revolutionizes LC analysis
Stainless steel components with the performance of PEEK - inert, robust and cost-effective

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.
Last viewed contents
Convective_condensation_level
Short_tandem_repeat
Traugott_Sandmeyer
Resampling_(statistics)
Atomic_emission_spectrum
Dumas_method
Meat_thermometer
Cardiac_stress_test
C3_carbon_fixation
Mode_shape