A water-based, rechargeable battery
Inexpensive and stable
water could form the basis for future, particularly inexpensive rechargeable batteries. Empa researchers have succeeded in doubling the electrochemical stability of water with a special saline solution. This takes us one step closer to using the technology commercially.
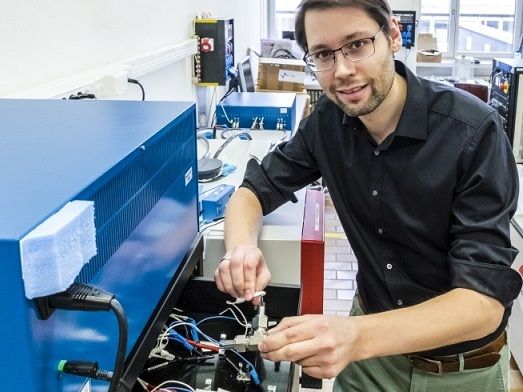
Research on the water electrolyte: Empa researcher Ruben-Simon Kühnel connecting a test cell to the charger with the concentrated saline solution. The stability of the system is determined in several charging and discharging cycles.
Empa
In the quest to find safe, low-cost batteries for the future, eventually we have to ask ourselves a question: Why not simply use water as an electrolyte? Water is cheap, in abundant supply, doesn’t burn and can conduct ions. But it has one major drawback: It is only chemically stable up to a voltage of 1.23 volts. In other words, a water cell provides three times less voltage than a customary lithium ion cell with 3.7 volts, which makes it poorly suited for applications in an electric car. A cost-effective, water-based battery, however, could be extremely interesting for stationary electricity storage applications.
Saline solution without free water
Ruben-Simon Kühnel and David Reber, researchers from Empa’s Materials for Energy Conversion Laboratory, have now discovered a way to potentially solve the problem: The saline electrolyte has to be liquid but so concentrated as not to contain any “surplus” water.
For their experiments, the two researchers used the special salt sodium FSI (precise name: sodium bis(fluorosulfonyl)imide). This salt dissolves extremely well in water: seven grams of sodium FSI and one gram of water produce a clear saline solution (see clip). As all the water molecules are clustered around the positively charged sodium cations in a hydrate envelope in this liquid, there are virtually no unbound water molecules left.
Cheap to produce
The researchers discovered that this saline solution displays an electrochemical stability of up to 2.6 volts – i.e. nearly twice as much as other aqueous electrolytes. The discovery could be the key to inexpensive, safe battery cells; inexpensive because, apart from anything else, the sodium FSI cells can be constructed more safely and thus more easily than the familiar lithium ion batteries.
The system has already withstood a series of charging and discharging cycles in the lab. Until now, however, the researchers have been testing the anodes and cathodes of their test battery separately – against a standard electrode as a partner. In the next step, the two half cells are to be combined into a single battery. Then additional charging and discharging cycles are scheduled.
Empa’s research activities on novel batteries for stationary electricity storage systems are embedded in the Swiss Competence Center for Heat and Electricity Storage (SCCER HaE), which coordinates research for new heat and electricity storage concepts on a national level and is led by the Paul Scherrer Institute (PSI). If the experiment succeeds, inexpensive water batteries will be within touching distance.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.