Novel MOF shell-derived surface modification of Li-rich layered oxide cathode
Rapid development of portable electronics and electric vehicles requires lithium-ion batteries (LIBs) to have high energy/power density, low cost, good safety and long lifespan. In a commercial LIB, the traditional cathode and anode materials are LiCoO2 and graphite, respectively. Compared with the commercial graphite (theoretical?capacity 372 mAh g-1), the LiCoO2 has a low specific capacity of 150 mAh g-1 which becomes a big bottleneck of the battery breakthroughs. Among numerous cathode materials, Li-rich layered oxide (LLO) materials have attracted increasing attention as promising candidates because of their high specific capacity (> 250 mAh g-1) and high operating voltage (> 3.5 V vs. Li+/Li). However, an undesired spinel growth in the layered host structure usually occurred from the surface during the long-term cycling, which led to the fast capacity fading and voltage decaying.
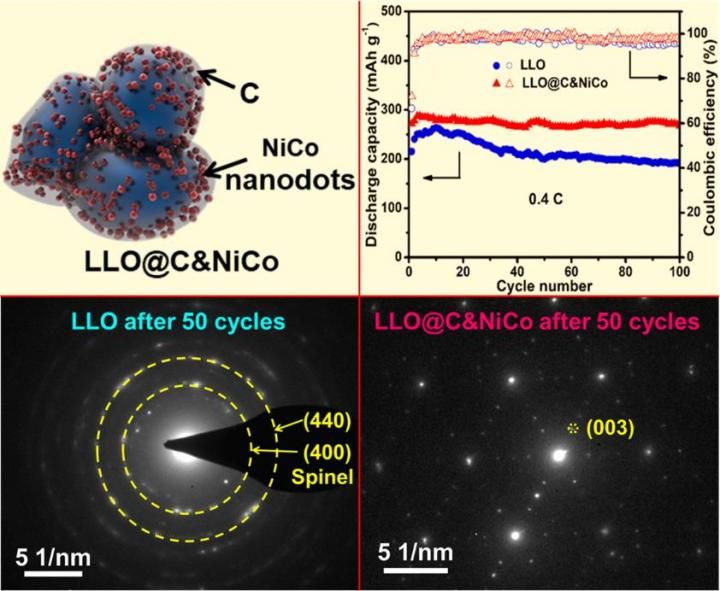
This is a schematic illustration of LLO@C&NiCo; Cycling performance and the corresponding Coulombic efficiencies tested at current densities of 0.4 C; SAED patterns of LLO and LLO@C&NiCo after 50 cycles at 0.4 C.
©Science China Press
Prof. L. Q. Mai has led a research team at Wuhan University of Technology aiming to improve the electrochemical performance of electrode materials. They have developed a facile and general carbon coating technology. The unique NiCo nanodots decorated carbon shell was constructed on the as-prepared Li1.2Mn0.54Ni0.13Co0.13O2 nanoparticles masterly on this basis. The obtained LLO@C&NiCo cathode exhibits enhanced cycling and rate capability with a capacity retention of 95% after 100 cycles at 0.4 C, 90% after 300 cycles at 2 C and a high capacity of 159 mAh g-1 at 5 C, respectively.
The in-situ X-ray diffraction, electrochemical impedance spectroscopy and selected area electron diffraction analyses after cycling demonstrate that the LLO@C&NiCo as a cathode material for LIBs exhibiting superior electrochemical performances, which is due to its unique protective C&NiCo shell. It promotes the electron conductivity (5 times), reduces the diffusion impedance, provides a robust structure for LLO which suppresses the undesired formation of the spinel phase initiated from the particle surface during cycling, and also protects the surface structure from side reactions at the electrode/electrolyte interface.
Original publication
Zhitong Xiao, Jiashen Meng, Qi Li, Xuanpeng Wang, Meng Huang, Ziang Liu, Chunhua Han, and Liqiang Mai; "Novel MOF shell-derived surface modification of Li-rich layered oxide cathode for enhanced lithium storage"; Science Bulletin; Available online 13 December 2017
Jiashen Meng, Xiong Liu, Jiantao Li, Qi Li, Chuan Zhao, Linhan Xu, Xuanpeng Wang, Fang Liu, Wei Yang, Xiaoming Xu, Ziang Liu, Chaojiang Niu, and Liqiang Mai; "General oriented synthesis of precise carbon-confined nanostructures by low-pressure vapor superassembly and controlled pyrolysis"; Nano letters
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.

Topic World Battery Technology
The topic world Battery Technology combines relevant knowledge in a unique way. Here you will find everything about suppliers and their products, webinars, white papers, catalogs and brochures.