Breakthrough in industrial-scale nanotube processing
Rice pioneers method for processing carbon nanotubes in bulk fluids
Advertisement
rice University scientists unveiled a method for the industrial-scale processing of pure carbon-nanotube fibers that could lead to revolutionary advances in materials science, power distribution and nanoelectronics. The result of a nine-year program, the method builds upon tried-and-true processes that chemical firms have used for decades to produce plastics. The research is available online in the journal Nature nanotechnology.

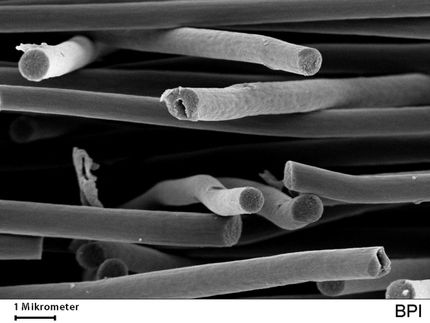
The liquid-crystaline phase of carbon nanotubes dissolved in chlorosulfonic acid.
Matteo Pasquali/Rice University
"Plastics is a $300 billion U.S. industry because of the massive throughput that's possible with fluid processing," said Rice's Matteo Pasquali, a paper co-author and professor in chemical and biomolecular engineering and in chemistry. "The reason grocery stores use plastic bags instead of paper and the reason polyester shirts are cheaper than cotton is that polymers can be melted or dissolved and processed as fluids by the train-car load. Processing nanotubes as fluids opens up all of the fluid-processing technology that has been developed for polymers."
The report was co-authored by an 18-member team of scientists from Rice's Richard E. Smalley Institute for Nanoscale Science and Technology, the University of Pennsylvania and the Technion-Israel Institute of Technology. Co-authors include Smalley Institute namesake Rick Smalley, the late Nobel laureate chemist who developed the first high-throughput method for producing high-quality carbon nanotubes, as well as Virginia Davis, a former doctoral student of Pasquali's and Smalley's who is now a professor at Auburn University, and Micah Green, a former postdoctoral researcher of Pasquali's who is now a professor at Texas Tech University.
The new process builds upon the 2003 Rice discovery of a way to dissolve large amounts of pure nanotubes in strong acidic solvents like sulfuric acid. The research team subsequently found that nanotubes in these solutions aligned themselves, like spaghetti in a package, to form liquid crystals that could be spun into monofilament fibers about the size of a human hair.
"That research established an industrially relevant process for nanotubes that was analogous to the methods used to create Kevlar from rodlike polymers, except for the acid not being a true solvent," said Wade Adams, director of the Smalley Institute and co-author of the new paper. "The current research shows that we have a true solvent for nanotubes -- chlorosulfonic acid -- which is what we set out to find when we started this project nine years ago."
Following the 2003 breakthrough with acid solvents, the team methodically studied how nanotubes behaved in different types and concentrations of acids. By comparing and contrasting the behavior of nanotubes in acids with the literature on polymers and rodlike colloids, the team developed both the theoretical and practical tools that chemical firms will need to process nanotubes in bulk.
"Ishi Talmon and his colleagues at Technion did the critical work required to help get direct proof that nanotubes were dissolving spontaneously in chlorosulfonic acid," Pasquali said. "To do this, they had to develop new experimental techniques for direct imaging of vitrified fast-frozen acid solutions."
Talmon said, "This was a very difficult study. Matteo's team not only had to pioneer new experimental techniques to achieve this, they also had to make significant extensions to the classical theories that were used to describe solutions of rods. The Technion team had to develop a new methodology to enable us to produce high-resolution images of the nanotubes dispersed in chlorosulfonic acid, a very corrosive fluid, by state-of-the-art electron microscopy at cryogenic temperatures."
Co-author Nicholas Parra-Vasquez, a Rice graduate student advised by Pasquali who is now working in France, said, "In looking at the project when I started, I had no idea where it was going to end up and how much work needed to be done. The project encompassed many students and professors, as well as collaborations with other schools. Because of this, it was a slow process but one that left no avenue unchecked. Looking on it now, I can't believe how big it became -- how much effort was put into every point found."
Few technological breakthroughs have been hyped as much as carbon nanotubes. Since their discovery in 1991, nanotubes have been touted as everything from a cure for cancer to a solution for the world's energy crisis. The hype is all the more remarkable given that nanotubes are notoriously difficult to work with and that chemists worldwide struggled for years even to make them.
So why the hype? Put simply, carbon nanotubes are remarkable. While they are roughly the same size and shape as some rodlike polymer molecules, nanotubes can conduct electricity as well as copper, and they can be either metals or semiconductors. They can be tagged with antibodies to diagnose diseases or heated with radio waves to destroy cancer. They've been used to make transistors far smaller than those in today's finest microchips. Nanotubes also weigh about one-sixth as much as steel but can be up to 100 times stronger.
"Kevlar, the polymer fiber used in bulletproof vests, is about five to 10 times stronger than our strongest nanotube fibers today, but in principle we should be able to make our fibers about 100 times stronger," Pasquali said. "If we can realize even 20 percent of our potential, we will have a great material, perhaps the strongest ever known.
"The electrical conductivity is already pretty good," he said. "It's about the same of the best-conducting carbon-carbon fibers, and that could be improved 200 times if better production methods for metallic nanotubes can be found."
The new research appears just as the Smalley Institute prepares for a 10th anniversary celebration Nov. 5 of the creation of Smalley's "HiPco" reactor, the first system capable of producing high-quality nanotubes in bulk. HiPco, short for high-pressure carbon monoxide process, broke the logjam on nanotube production and cleared the way for more scientific study and for industry to begin using them in some materials. Industrial nanotube reactors today generate several tons of low-quality carbon nanotubes per year, and the worldwide market for nanotubes is expected to top $2 billion annually within the next decade.
But a final breakthrough remains before the true potential of high-quality carbon nanotubes can be realized. That's because HiPco and all other methods of making high-end, "single-walled" nanotubes generate a hodgepodge of nanotubes with different diameters, lengths and molecular structures. Scientists worldwide are scrambling to find a process that will generate just one kind of nanotube in bulk, like the best-conducting metallic varieties, for instance.
"One good thing about the process that we have right now is that if anybody could give us one gram of pure metallic nanotubes, we could give them one gram of fiber within a few days," Pasquali said.