Scientists to use artificial photosynthesis and nanotubes to generate hydrogen fuel with sunlight
US Department of Enregy awards $1.7 million to explore new 'green' energy creation
A team of four chemists at the University of Rochester have begun work on a new kind of system to derive usable hydrogen fuel from water using only sunlight.
The project has caught the attention of the U.S. Department of Energy, which has just given the team nearly $1.7 million to pursue the design.
"Everybody talks about using hydrogen as a super-green fuel, but actually generating that fuel without using some other non-green energy in the process is not easy," says Kara Bren, professor in the Department of Chemistry. "People have used sunlight to derive hydrogen from water before, but the trick is making the whole process efficient enough to be useful."
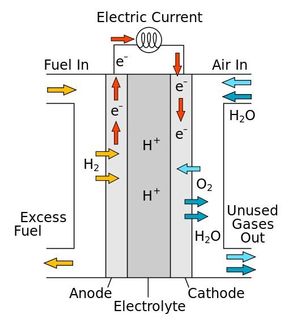
Bren and the rest of the Rochester team—Professor of Chemistry Richard Eisenberg, and Associate Professors of Chemistry Todd Krauss, and Patrick Holland—will be investigating artificial photosynthesis, which uses sunlight to carry out chemical processes much as plants do. What makes the Rochester approach different from past attempts to use sunlight to produce hydrogen from water, however, is that the device they are preparing is divided into three "modules" that allow each stage of the process to be manipulated and optimized far more easily than other methods.
The first module uses visible light to create free electrons. A complex natural molecule called a chromophore that plants use to absorb sunlight will be re-engineered to efficiently generate reducing electrons.
The second module will be a membrane suffused with carbon nanotubes to act as molecular wires so small that they are only one-millionth the thickness of a human hair. To prevent the chromophores from re-absorbing the electrons, the nanotube membrane channels the electrons away from the chromophores and toward the third module.
In the third module, catalysts put the electrons to work forming hydrogen from water. The hydrogen can then be used in fuel cells in cars, homes, or power plants of the future.
By separating the first and third modules with the nanotube membrane, the chemists hope to isolate the process of gathering sunlight from the process of generating hydrogen. This isolation will allow the team to maximize the system's light-harvesting abilities without altering its hydrogen-generation abilities, and vice versa. Bren says this is a distinct advantage over other systems that have integrated designs because in those designs a change that enhances one trait may degrade another unpredictably and unacceptably.
Bren says it may be years before the team has a system that clearly works better than other designs, and even then the system would have to work efficiently enough to be commercially viable. "But if we succeed, we may be able to not only help create a fuel that burns cleanly, but the creation of the fuel itself may be clean."
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.