Plastics that convert light to electricity could have a big impact
Researchers the world over are striving to develop organic solar cells that can be produced easily and inexpensively as thin films that could be widely used to generate electricity.
But a major obstacle is coaxing these carbon-based materials to reliably form the proper structure at the nanoscale (tinier than 2-millionths of an inch) to be highly efficient in converting light to electricity. The goal is to develop cells made from low-cost plastics that will transform at least 10 percent of the sunlight that they absorb into usable electricity and can be easily manufactured.
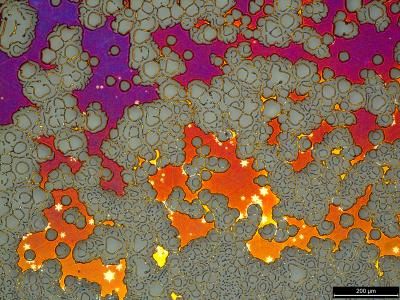
A research team headed by David Ginger, a University of Washington associate professor of chemistry, has found a way to make images of tiny bubbles and channels, roughly 10,000 times smaller than a human hair, inside plastic solar cells. These bubbles and channels form within the polymers as they are being created in a baking process, called annealing, that is used to improve the materials' performance.
The researchers are able to measure directly how much current each tiny bubble and channel carries, thus developing an understanding of exactly how a solar cell converts light into electricity. Ginger believes that will lead to a better understanding of which materials created under which conditions are most likely to meet the 10 percent efficiency goal.
As researchers approach that threshold, nanostructured plastic solar cells could be put into use on a broad scale, he said. As a start, they could be incorporated into purses or backpacks to charge cellular phones or mp3 players, but eventually they could make in important contribution to the electrical power supply.
Most researchers make plastic solar cells by blending two materials together in a thin film, then baking them to improve their performance. In the process, bubbles and channels form much as they would in a cake batter. The bubbles and channels affect how well the cell converts light into electricity and how much of the electric current actually gets to the wires leading out of the cell. The number of bubbles and channels and their configuration can be altered by how much heat is applied and for how long.
The exact structure of the bubbles and channels is critical to the solar cell's performance, but the relationship between baking time, bubble size, channel connectivity and efficiency has been difficult to understand. Some models used to guide development of plastic solar cells even ignore the structure issues and assume that blending the two materials into a film for solar cells will produce a smooth and uniform substance. That assumption can make it difficult to understand just how much efficiency can be engineered into a polymer, Ginger said.
For the current research, the scientists worked with a blend of polythiophene and fullerene, model materials considered basic to organic solar cell research because their response to forces such as heating can be readily extrapolated to other materials. The materials were baked together at different temperatures and for different lengths of time.
Ginger is the lead author of a paper documenting the work, published online last month by the American Chemical Society journal Nano Letters and scheduled for a future print edition. Coauthors are Liam Pingree and Obadiah Reid of the UW. The research was funded by the National Science Foundation and the U.S. Department of Energy.
Ginger noted that the polymer tested is not likely to reach the 10 percent efficiency threshold. But the results, he said, will be a useful guide to show which new combinations of materials and at what baking time and temperature could form bubbles and channels in a way that the resulting polymer might meet the standard.
Such testing can be accomplished using a very small tool called an atomic force microscope, which uses a needle similar to the one that plays records on an old-style phonograph to make a nanoscale image of the solar cell. The microscope, developed in Ginger's lab to record photocurrent, comes to a point just 10 to 20 nanometers across. The tip is coated with platinum or gold to conduct electrical current, and it traces back and forth across the solar cell to record the properties.
As the microscope traces back and forth over a solar cell, it records the channels and bubbles that were created as the material was formed. Using the microscope in conjunction with the knowledge gained from the current research, Ginger said, can help scientists determine quickly whether polymers they are working with are ever likely to reach the 10 percent efficiency threshold.
Making solar cells more efficient is crucial to making them cost effective, he said. And if costs can be brought low enough, solar cells could offset the need for more coal-generated electricity in years to come.
"The solution to the energy problem is going to be a mix, but in the long term solar power is going to be the biggest part of that mix," he said.
Most read news
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Making batteries from waste glass bottles - Researchers are turning glass bottles into high performance lithium-ion batteries
Ultra-low temperature freezing of research material
BASF and Gazprom intensify their cooperation
BASF expands production capacities for UV/EB acrylates
Bio-inspired catalysts that work in water open door to greener chemical processes
LyondellBasell Names Milton Carroll, Bruce A. Smith and Rudy van der Meer to Supervisory Board
Air Liquide Announces SARIS(TM), a New Balazs(TM) Analytical Service for Solid Materials Characterization