Element 112 shall be named "copernicium"
Proposed name honors astronomer Nicolaus Copernicus
Advertisement
In honor of scientist and astronomer Nicolaus Copernicus (1473-1543), the discovering team around Professor Sigurd Hofmann suggested the name "copernicium" with the element symbol "Cp" for the new element 112, discovered at the GSI Helmholtzzentrum für Schwerionenforschung (Center for Heavy Ion Research) in Darmstadt. It was Copernicus who discovered that the Earth orbits the Sun, thus paving the way for our modern view of the world.
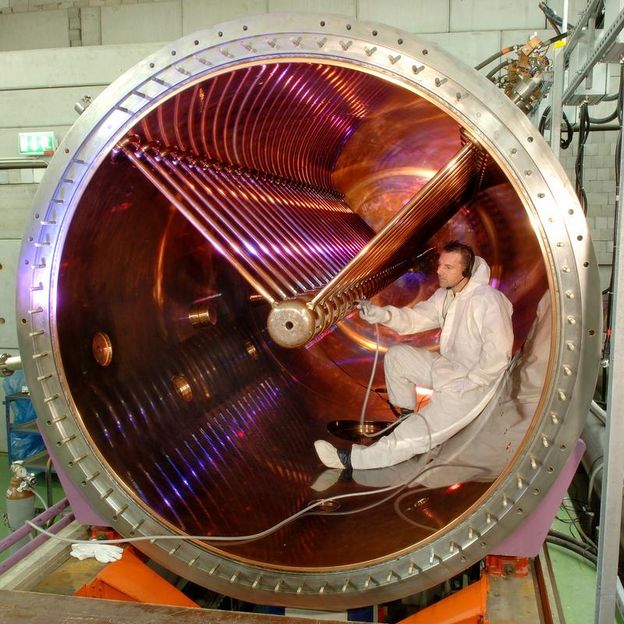
View inside the linear accelerator at GSI with a total length of 120 meters.
G. Otto, GSI
Thirteen years ago, element 112 was discovered by an international team of scientists at the GSI accelerator facility. A few weeks ago, the International Union of Pure and Applied Chemistry, IUPAC, officially confirmed their discovery. In around six months, IUPAC will officially endorse the new element's name. This period is set to allow the scientific community to discuss the suggested name "copernicium" before the IUPAC naming.
"After IUPAC officially recognized our discovery, we - that is all scientists involved in the discovery - agreed on proposing the name "copernicium" for the new element 112. We would like to honor an outstanding scientist, who changed our view of the world", says Sigurd Hofmann, head of the discovering team.
Copernicus was born 1473 in Torun; he died 1543 in Frombork, Poland. Working in the field of astronomy, he realized that the planets circle the Sun. His discovery refuted the then accepted belief that the Earth was the center of the universe. His finding was pivotal for the discovery of the gravitational force, which is responsible for the motion of the planets. It also led to the conclusion that the stars are incredibly far away and the universe inconceivably large, as the size and position of the stars does not change even though the Earth is moving. Furthermore, the new world view inspired by Copernicus had an impact on the human self-concept in theology and philosophy: humankind could no longer be seen as the center of the world.
With its planets revolving around the Sun on different orbits, the solar system is also a model for other physical systems. The structure of an atom is like a microcosm: its electrons orbit the atomic nucleus like the planets orbit the Sun. Exactly 112 electrons circle the atomic nucleus in an atom of the new element "copernicium".
Element 112 is the heaviest element in the periodic table, 277 times heavier than hydrogen. It is produced by a nuclear fusion, when bombarding zinc ions onto a lead target. As the element already decays after a split second, its existence can only be proved with the help of extremely fast and sensitive analysis methods. Twenty-one scientists from Germany, Finland, Russia and Slovakia have been involved in the experiments that led to the discovery of element 112.
Since 1981, GSI accelerator experiments have yielded the discovery of six chemical elements, which carry the atomic numbers 107 to 112. The discovering teams at GSI already named five of them: element 107 is called bohrium, element 108 hassium, element 109 meitnerium, element 110 darmstadtium, and element 111 is named roentgenium.