Making nanoparticles in artificial cells
Two new construction manuals are now available for the world's smallest lamps. Based on these protocols, scientists from the Max Planck Institute of colloids and Interfaces have tailor-made nanoparticles that can be used as position lights on cell proteins and, possibly in the future as well, as light sources for display screens or for optical information technology. The researchers produced cadmium sulphide particles in microscopically small membrane bubbles. Depending on which of the construction manuals they follow, the particles can be 4 or 50 nanometres in size. Because the membrane bubbles have the same size as living cells, the scientists' work also provides an indication as to how nanostructures could arise in nature.
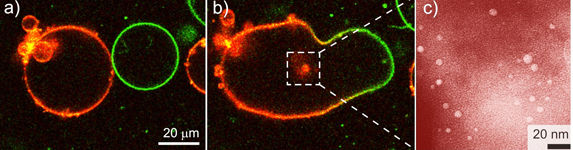
Vesicles with different reactants have different fluorescent substances in their membranes (a). When the bubbles fuse, red fluorescent nanoparticles form (b). The particles can be seen as bright dots under the transmission electron microscope (c).
MPI für Kolloid- und Grenzflächenforschung
Cells and microorganisms are absolute masters when it comes to working in the smallest possible dimensions. Like particularly efficient micro-factories, they produce particles and structures from inorganic material, for example pieces of chalk, that are only a few nanometres in size, that is, millionths of a millimetre. Cells have two different factors to thank for this capability. First, they have peptides, a biological tool at their disposal that may shape the chalk into a desired form. Second, the fact that they are very small themselves is convenient: the chalk particles cannot grow boundlessly - the end is reached when the calcium carbonate, the building block of chalk, runs out in the cell.
"We used the fact that cells represent a closed reaction container as a model for the synthesis of nanoparticles," says Rumiana Dimova. Her group at the Max Planck Institute of Colloids and Interfaces studies membranes - the cell envelope. The scientist and her colleagues form bubbles that are around 50 micrometres in size from lecithin membranes, which are similar to biological membranes. Like cells, membrane bubbles - or vesicles as scientists refer to them - also provide a closed reaction container. The scientists load the membrane bubbles with one of two reactants for the nanoparticles.
From this point, the researchers have developed two different sets of protocols. In one case, they produce bubbles loaded with one of the two reactants, sodium sulphide or cadmium chloride. The scientists then bring the bubbles with the different loads together and fuse two vesicles to form a bigger vesicle - this is done by subjecting the bubble cocktail to a short but very strong electrical pulse. The electric shock fuses the membranes of two adjacent bubbles.
In many cases, this results in the fusion of two bubbles containing different reactants. These then react to form cadmium sulphide, which is not water soluble and thus precipitates in the form of nanoparticles. "Because the reactants are only present to a limited extent in the fused bubbles, the particles only grow to a size of four nanometres," explains Rumiana Dimova. The scientists were able to track the entire process directly under the microscope because they had added different fluorescent molecules to the membranes of the differently loaded vesicles. The researchers were also able to see the nanoparticles forming as the particles shone like tiny lamps.
In the second process, the researchers only produce vesicles with one of the reactants. When the vesicles have formed, unlike in the first procedure, the researchers do not remove them from the production chamber. Instead, the bubbles remain attached to their substrate via small membrane channels, like balloons tied to strings, and stand in a solution that is the same as the one inside them. The researchers working with Rumiana Dimova then altered this situation: they substituted the solution with the first ingredient for the nanoparticles with a second component. This causes no change inside the vesicles at first. The second ingredient only creeps gradually between the substrate and membrane into the channel and to the vesicle. In the vesicle, where the other ingredient is already waiting, the nanoparticles grow again - this time to a size of 50 nanometres.
"With our method, we succeeded for the first time in producing particles with a certain diameter in vesicles whose size corresponds to that of cells," says Rumiana Dimova. Previously, biologists thought that cells depended on the help of peptides for the synthesis of nanoparticles. However, as Rumiana Dimova and her colleagues have discovered, it can also be done without them.
Original publication: Peng Yang, Reinhard Lipowsky, and Rumiana Dimova; "Nanoparticle Formation in Giant Vesicles: Synthesis in Biomimetic Compartments"; Small, published online, June 8, 2009
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.