Controlling zeolite morphologies
Scientists in Korea control the morphology of zeolites using a structure-directing agent, leading to further understanding of the mechanism involved.
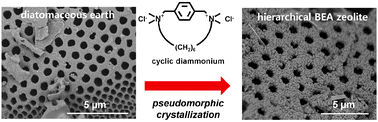
Zeolites are widely used as catalysts and absorbents due to their crystalline microporous structures. The aluminosilicate structures can be selectively synthesised with uniform pore diameters in the range of 0.3 to 0.8 nm. However this can lead to diffusion limitations in application as the pore size is often too small. These diffusion limitations can often be removed by using mesoporous structures with pore sizes of 2 to 20 nm in diameter.
Ryong Ryoo and colleagues at the Korea Advanced Institute of Science and Technology, in Daejeon, have prepared zeolites with hierarchically mesoporous-microporous structures using a cyclic quaternary diammonium compound (CD) as a structure-directing agent (SDA).
Significantly, the SDA can effectively solidify silicate ions into a mesoporous gel structure. The silicate mobility is suppressed in the presence of the SDA leading to crystallisation of the zeolite without significant morphological change from the mesoporous gel.
‘The use of CD sheds meaningful light to the synthesis mechanism and may lead to a general synthesis methodology to various zeolites with controlled morphologies,’ says Ryoo.
Ryoo and his team hope that these mesoporous-microporous zeolites will show improved catalytic properties such as selectivity, activity and catalyst lifetime, when compared with ordinary zeolite crystals, due to facile molecular diffusion through the meospores.
Ryoo suggests that the next challenge in this research area is to rationally design various CD-like molecules as SDAs, that possess sufficiently high chemical stabilities under zeolite crystallisation conditions.
Original article: Ryoo et. al.; "The synthesis of a hierarchically porous BEA zeolite via pseudomorphic crystallization"; Chem. Commun. 2009
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.