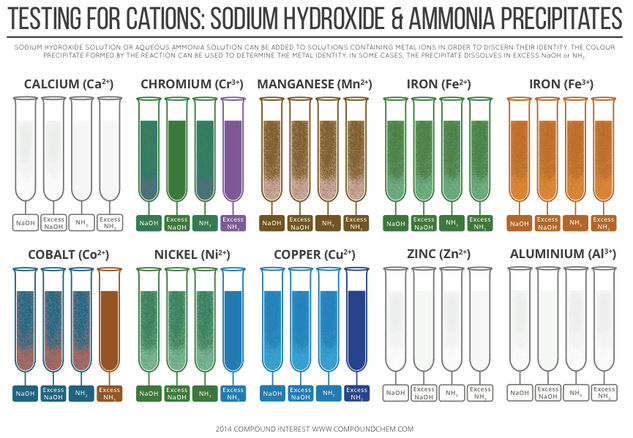
© Compound InterestTesting for Cations – Sodium Hydroxide & Ammonia Precipitates
A previous post looked at the colours of transition metals, and the origin of their colours – this graphic, on the other hand, looks at how transition metals (and some non-transition metals) can be identified by the precipitates they form with sodium hydroxide and ammonia solutions. I’m going to keep the explanation of the reasons for the colour changes and precipitates fairly simple here, but I’ve provided links at the bottom of the page if you want to read about them in more detail.
In the case of the d block metals (all commonly referred to as transition metals, although according to the definition zinc is not a transition metal), the reactions of their aqueous ions with sodium hydroxide and ammonia solutions are due, in some cases, to ligand exchange reactions. The d block metals form metal aquo complexes, like that shown above.