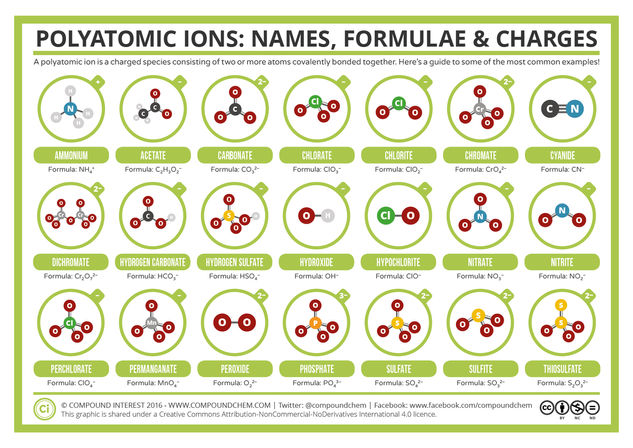
© Compound InterestCommon Polyatomic Ions: Names, Formulae, and Charges
Know your sulfates from your sulfites, and your chlorates from your perchlorates? This graphic gives a helping hand with remembering the names, formulae and charges of various different polyatomic ions. The selection covers all of the ions GCSE students are likely to come across, as well as the vast majority of those that will be encountered by A level students.
The structures of the ions are shown with dashed bonds to indicate where the charge is delocalised across the ion. The charge itself is shown by the surrounding circle. I’m aware that this manner of drawing them, showing the charge delocalised, might be somewhat unfamiliar to GCSE and A level students, but as it’s the more correct way of showing them, I decided to stick with it. However, if there’s interest in an alternate version with an example resonance structure shown for each ion, then I can probably make that happen.
As far as the ions included go, there are obviously some that miss out and aren’t included, but I’ve tried to make these ones that wouldn’t normally be encountered by school chemistry students anyway. An omission that’s already bugging me a little is that of the iodate ion, simply because I know that A level students will encounter that one – however, the analogous chlorate ion is included. Again, if there’s interest, I’m already considering expanding this one to include ALL the polyatomic ions (or, at least, as many as possible)!