© Compound InterestSodium Hypochlorite
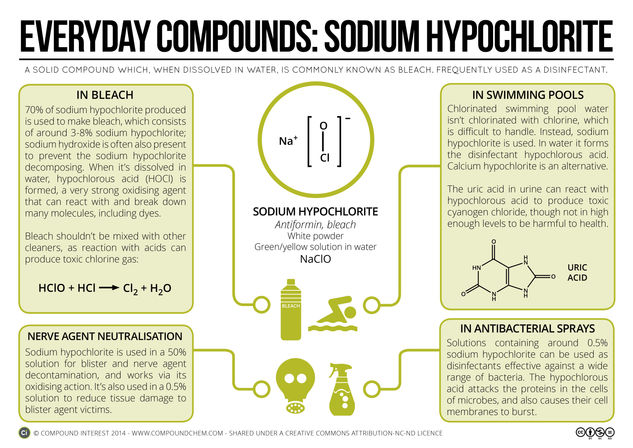
It’s been a little while since the last entry in the Everyday Compounds series, so today’s post takes a look at Sodium Hypochlorite. This chemical is likely to be found in several cleaning products in your kitchen, and additionally is one of the main compounds used to chlorinate the water in swimming pools. Here’s a look at the chemistry behind these uses, and the potential dangers.
Sodium hypochlorite is a solid white powder, but is more commonly used dissolved in water. Solutions of sodium hypochlorite are commonly referred to as bleach, although household bleach also contains small amounts of several other compounds, including sodium hydroxide and calcium hypochlorite. Sodium hypochlorite generally makes up 3-8% of the volume; dissolved in water, it has a strongly alkaline pH, which can irritate the skin. The idea of strong acids causing burns is common knowledge, but in fact, strong alkalis can be just as dangerous, and concentrated bleach is at a high enough pH to cause burns to the skin on contact.