© Compound InterestAcetic Acid – Vinegar & Volcanoes
It’s been a little while since the last post in the ‘Everyday Chemicals’ series, but it’s back today, and with a revamped look (which will also be applied out to the previous posts in the series over the next week or so). The latest post looks at acetic acid; this compound is well known for its presence in vinegar, but has a role in the manufacture of other chemicals we regularly encounter. It’s also an important part of a classic home science experiment!
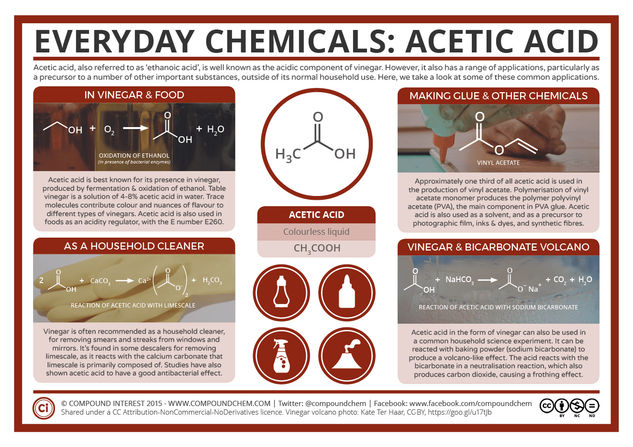
Acetic acid also has the name ethanoic acid, though this is less commonly used. Most people will know of its presence in vinegar, from which its name is derived – it originates from the latin word for vinegar. In vinegar acetic acid is generated by fermentation, which produces ethanol, and then subsequent oxidation of this ethanol. The method of acetic acid’s production by the oxidation of ethanol also helps explain why wines can begin to taste vinegary if the bottle is left open.