© Compound InterestThe Chemistry of Poinsettia Plants
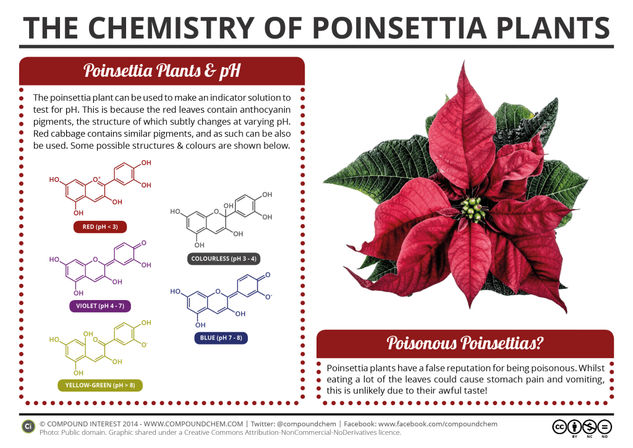
Following on from the start of the Chemistry Advent Calendar yesterday, here’s another festive post, this time looking at the chemistry of the poinsettia plant. The red leaves of the poinsettia plant can be used to make a pH indicator, due to their chemical composition; this is actually something of an upgrade on one of the oldest posts on the site, now complete with a explanatory graphic!
You’ll have almost certainly learnt about pH in chemistry lessons, be that recently or otherwise. In case you’re in need of a reminder, pH is a scale that allows us to compare the acidity or basicity of solutions. More specifically, it’s determined by the concentration of hydrogen ions in solution; the greater the concentration, the lower the pH and the more acidic the solution is. A number of different indicators are available to test for the pH of a solution, but you can also make a pH indicator from the leaves of the poinsettia plant.