© Compound InterestThe Chemistry of Candy
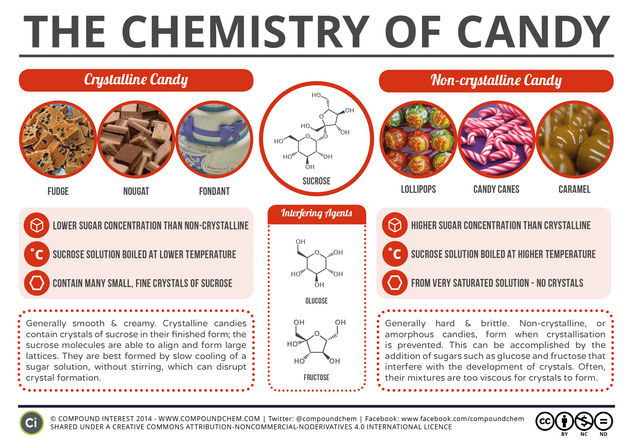
In this graphic, we look at the amazing versatility of sucrose, and how (combined with other ingredients) it can make candies as hard as lollipops, or as soft as fudge.
Whilst there are a huge variety of candies available, we can actually divide them into just two main categories: crystalline and non-crystalline (or amorphous). These designations are derived from the arrangement of the sucrose molecules within the candy, which is deliberately controlled during the candy-making process. Before we consider these categories, though, we need to consider how candy is made. The sugar will be mixed with the other required ingredients and water, then heated to a desired boiling temperature which influences the final sugar concentration. It is then allowed to cool – and it is the difference in the processes that occur during the cooling which dictate the type of candy formed.